|
Organogallium Chemistry
Organogallium chemistry is the chemistry of organometallic compounds containing a carbon to gallium (Ga) chemical bond. Despite their high toxicity , organogallium compounds have some use in organic synthesis. The compound trimethylgallium is of some relevance to MOCVD as a precursor to gallium arsenide via its reaction with arsine at 700 °C: :Ga(CH3)3 + AsH3 → GaAs + 3CH4 Gallium trichloride is an important reagent for the introduction of gallium into organic compounds. The main gallium oxidation state is Ga(III), as in all lower group 13 elements (such as aluminium). Organogallium(III) chemistry Compounds of the type R3Ga are monomeric. Lewis acidity decreases in the order Al > Ga > In and as a result organogallium compounds do not form bridged dimers as organoaluminum compounds do. Organogallium compounds are also less reactive than organoaluminum compounds. They do form stable peroxides. Organogallium compounds can be synthesized by transmetallation, for example the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmetallation
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form: :M1–R + M2–R′ → M1–R′ + M2–R where R and R′ can be, but are not limited to, an alkyl, aryl, alkynyl, allyl, halogen, or pseudohalogen group. The reaction is usually an irreversible process due to thermodynamic and kinetic reasons. Thermodynamics will favor the reaction based on the electronegativities of the metals and kinetics will favor the reaction if there are empty orbitals on both metals. There are different types of transmetalation including redox-transmetalation and redox-transmetalation/ligand exchange. During transmetalation the metal-carbon bond is activated, leading to the formation of new metal-carbon bonds. Transmetalation is commonly used in catalysis, synthesis of main group complexes, and synthesis of transition metal complexes. Types of transmetalation There are two main types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienylindium(I)
Cyclopentadienylindium(I), C5H5In, is an organoindium compound containing indium in the +1 oxidation state. Commonly abbreviated to CpIn, it is a cyclopentadienyl complex with a half-sandwich structure. It was the first (1957) low valent organoindium compound reported. Preparation and chemistry CpIn can be readily prepared by reacting indium(I) chloride with cyclopentadienyllithium: :InCl + CpLi → CpIn + LiCl InCp reacts with BF3, BCl3, BBr3, BI3 and trimethylborane B(CH3)3 to form adducts, e.g.: :CpIn + BF3 → CpIn·BF3 In these adducts the bonding of the Cp ligand to the indium atom changes from η5 (π complexing) to η1 (σ bonding). Salts containing the InX2− anion containing indium in the +1 oxidation state have been prepared, for example: :CpIn + HCl + N(Et4)Cl → N(Et4)InCl2+ C5H6 Structure and bonding Solid CpIn is polymeric consisting of zigzag chains of alternating indium atoms and C5H5 units. Two indium atoms interact with the opposite faces of ea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalysis, catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze Ziegler–Natta catalyst, olefin polymerization. Some metallocenes consist of metal plus two cyclooctatetraenide anions (, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others). Metallocenes are a subset of a broader class of compounds called sandwich compounds. In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromaticity, aromatically st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thallium
Thallium is a chemical element with the Symbol (chemistry), symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek language, Greek , , meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year. Thallium tends to form the +3 and +1 oxidation states. The +3 state resembles that of the other elements in Boron Group, group 13 (boron, aluminium, galli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year. Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoindium Chemistry
Organoindium chemistry is the chemistry of compounds containing In-C bonds. The main application of organoindium chemistry is in the preparation of semiconducting components for microelectronic applications. The area is also of some interest in organic synthesis. Most organoindium compounds feature the In(III) oxidation state, akin to its lighter congeners Ga(III) and B(III). 166px, Trimethylindium Organoindium(I) Monovalent In is relatively more common than Ga(I) or B(I). One example is cyclopentadienylindium(I). Organoindium(III) Trimethylindium is a colorless, volatile solid. It is the preferred source of indium for metalorganic vapour phase epitaxy ( MOVPE) of indium-containing compound semiconductors, such as InP, InAs, AlInGaNP, etc. InMe3 is pyrophoric. To obtain the trialkyl derivatives, alkylation of indium trihalides with organolithium reagents is typical. OrganoIn(III) compounds are also prepared by treating In metal with alkyl halides. This reaction g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
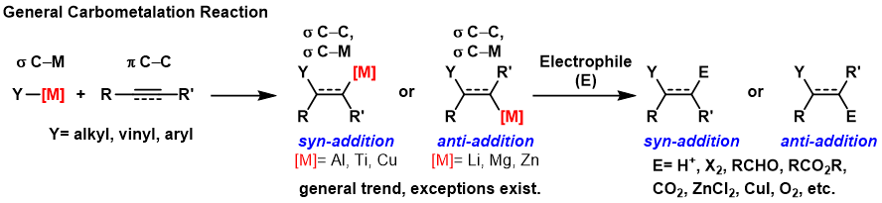
Carbometallation
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oligomerization or polymerization see Ziegler-Natta polymerization) or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometalations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the ''anti''-addition product with high selectivity and some yield the syn-addition product. The outcome of ''syn'' and ''anti''- addition products is determined by the mechanism of the carbometalation. Carboalumination The carboalumination reaction is most commonly catalyzed by zirconocene dichloride (or related catalyst). Some carboaluminat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barbier Reaction
The Barbier reaction is an organometallic reaction between an alkyl halide (chloride, bromide, iodide), a carbonyl group and a metal. The reaction can be performed using magnesium, aluminium, zinc, indium, tin, samarium, barium or their salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the organometallic species in the Barbier reaction is generated ''in situ'', whereas a Grignard reagent is prepared separately before addition of the carbonyl compound. Unlike many Grignard reagents, the organometallic species generated in a Barbier reaction are unstable and thus cannot be stored or sold commercially. Barbier reactions are nucleophilic addition reactions that involve relatively inexpensive, water insensitive metals (e.g zinc powder) or metal compounds. For this reason it is possible in many cases to run the reaction in water, making the procedure part of green chemistry. In cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dewar–Chatt–Duncanson Model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry that explains the chemical bonding in transition metal alkene complexes. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A. Duncanson. The alkene donates electron density into a π-acid metal d-orbital from a π-symmetry bonding orbital between the carbon atoms. The metal donates electrons back from a (different) filled d-orbital into the empty π* antibonding orbital. Both of these effects tend to reduce the carbon-carbon bond order, leading to an elongated C−C distance and a lowering of its vibrational frequency. In Zeise's salt K platinum">PtCl3(C2H4).html" ;"title="platinum.html" ;"title="/nowiki>platinum">PtCl3(C2H4)">platinum.html" ;"title="/nowiki>platinum">PtCl3(C2H4)sup>.H2O the C−C bond length has increased to 134 picometres from 133 pm for ethylene. In the nickel compound Ni(C2H4)(PPh3)2 the value is 143 pm. The interaction also causes carbon atoms to "rehybridise" from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex Formation
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



-aspicillin.png)


