|
Nef Isocyanide Reaction
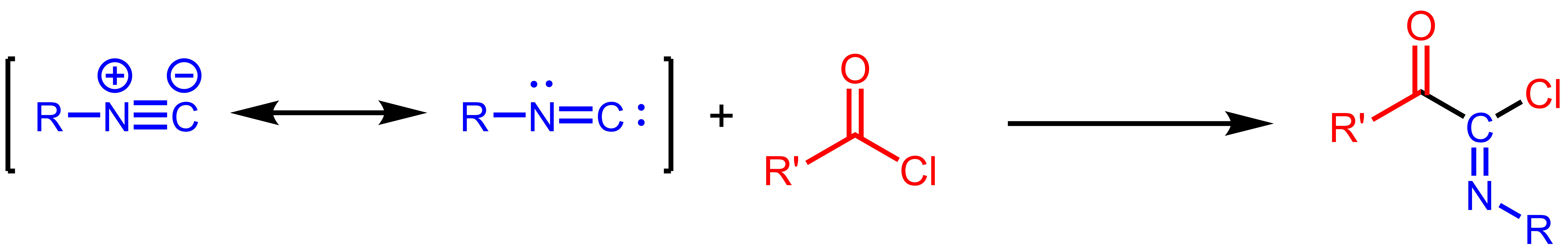
The Nef isocyanide reaction is an addition reaction that takes place between isocyanides and acyl chlorides to form imidoyl chloride products, a process first discovered by John Ulrich Nef. The product imidoyl chloride can be hydrolyzed to give the amide, trapped with other nucleophiles, or undergo halide abstraction with silver salts to form an acyl nitrilium intermediate. The reaction is of some theoretical interest, as kinetic measurements and DFT studies have indicated that the addition occurs in one step, without the intermediacy of a tetrahedral intermediate that is commonly proposed for carbonyl addition reactions. See also * Carbene * Insertion reaction * Nef reaction In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane () to an aldehyde () or a ketone () and nitrous oxide (). The reaction has been the subject of several ... References {{reflist Carbon-carbon bond formin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct).. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon–carbon double bonds (alkenes), or with triple bonds (alkynes), and compounds that have rings, which are also considered points of unsaturation. Molecules containing carbon— hetero double bonds like carbonyl () groups, or imine () groups, can undergo addition, as they too have double-bond character. An addition reaction is the reverse of an elimination reaction. For instance, the hydration of an alkene to an alcohol is reversed by dehydration. There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions exist as well, called free-radical addition and cycloadditions. Addition reactions are also encountered in polymerizations and called a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful representations. They are sus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : When other functional groups take priority, acyl chlorides are considered prefixes — ''chlorocarbonyl-'': : Properties Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1. The simplest s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
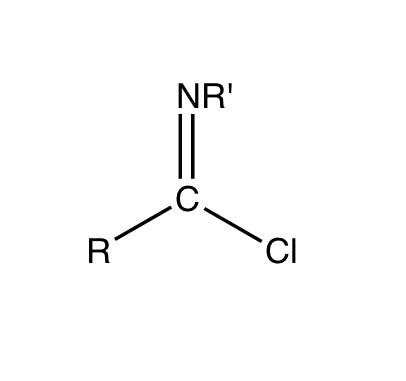
Imidoyl Chloride
Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond.Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; pp. 55–112. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidines. Synthesis and properties Imidoyl halides are synthesized by combining amides and halogenating agents. The structure of the carboxylic acid amides plays a role in the outcome of the synthesis. Imidoyl chloride can be prepared b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Ulric Nef (chemist)
John Ulric Nef (Johann Ulrich Nef; June 14, 1862 – August 13, 1915) was a Swiss-born American chemist and the discoverer of the Nef reaction and Nef synthesis. He was a member of the American Academy of Arts and Sciences and the National Academy of Sciences. Life His parents emigrated from Switzerland to the United States, where Nef studied chemistry at Harvard University until 1884. Upon graduation, he joined Adolf von Baeyer at the University of Munich, where he received his Ph.D. in 1887. He was a professor at Purdue University from 1887 till 1889 and at Clark University from 1889 till 1892. In 1892 Nef joined the newly formed University of Chicago as Professor of Chemistry, where he spent the rest of his academic career. His son John Ulric Nef (1899–1988) became a Professor of Economic History and published several books. Work The discovery of the Nef reaction and the papers about divalent carbon (carbenes) were his major achievements. See also *Phenylsodium Phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets, depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well-studied carbene is dichlorocarbene , which can be generated ''in situ'' from chloroform and a strong base. Structures and bonding The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. Most carbenes have a nonlinear triplet ground state, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insertion Reaction
An insertion reaction is a chemical reaction where one chemical entity (a molecule or molecular fragment) interposes itself into an existing bond of typically a second chemical entity ''e.g.'': : + \longrightarrow The term only refers to the result of the reaction and does not suggest a mechanism. Insertion reactions are observed in organic, inorganic, and organometallic chemistry. In cases where a metal-ligand bond in a coordination complex is involved, these reactions are typically organometallic in nature and involve a bond between a transition metal and a carbon or hydrogen. It is usually reserved for the case where the coordination number and oxidation state of the metal remain unchanged. When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination. There are two common insertion geometries— 1,1 and 1,2 (pictured above). Additionally, the inserting molecule can act either as a nucleophile or a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nef Reaction
In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane () to an aldehyde () or a ketone () and nitrous oxide (). The reaction has been the subject of several literature reviews. The reaction was reported in 1894 by the chemist John Ulric Nef, who treated the sodium salt of nitroethane with sulfuric acid resulting in an 85–89% yield of nitrous oxide and at least 70% yield of acetaldehyde. However, the reaction was pioneered a year earlier in 1893 by Konovalov, who converted the potassium salt of 1-phenylnitroethane with sulfuric acid to acetophenone. Reaction mechanism The reaction mechanism starting from the nitronate salt as the resonance structures 1a and 1b is depicted below: The salt is protonated forming the nitronic acid 2 (in some cases these nitronates have been isolated) and once more to the iminium ion 3. This intermediate is attacked by water in a nucleophilic addition for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reactions
Addition (usually signified by the plus symbol ) is one of the four basic operations of arithmetic, the other three being subtraction, multiplication and division. The addition of two whole numbers results in the total amount or '' sum'' of those values combined. The example in the adjacent image shows a combination of three apples and two apples, making a total of five apples. This observation is equivalent to the mathematical expression (that is, "3 ''plus'' 2 is equal to 5"). Besides counting items, addition can also be defined and executed without referring to concrete objects, using abstractions called numbers instead, such as integers, real numbers and complex numbers. Addition belongs to arithmetic, a branch of mathematics. In algebra, another area of mathematics, addition can also be performed on abstract objects such as vectors, matrices, subspaces and subgroups. Addition has several important properties. It is commutative, meaning that the order of the operands d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |