Insertion Reaction on:
[Wikipedia]
[Google]
[Amazon]
An insertion reaction is a
 Mechanistically, the α-diazoketone undergoes a
Mechanistically, the α-diazoketone undergoes a  Perhaps surprisingly, α-bromoacetophenone is the minor product when this reaction is carried out with
Perhaps surprisingly, α-bromoacetophenone is the minor product when this reaction is carried out with  The
The 
 Here, the carbene intermediate inserts into a carbon-hydrogen bond to form the carbon-carbon bond needed to close the cyclopentene ring. Carbene insertions into carbon-hydrogen bonds can also occur intermolecularly:
:
Here, the carbene intermediate inserts into a carbon-hydrogen bond to form the carbon-carbon bond needed to close the cyclopentene ring. Carbene insertions into carbon-hydrogen bonds can also occur intermolecularly:
: Carbenoids are
Carbenoids are 
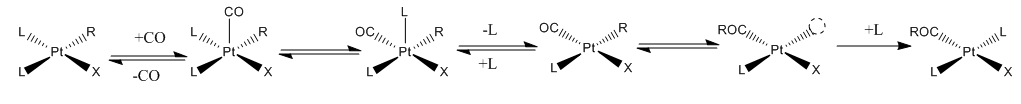 Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but
Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but  The Cativa process
The Cativa process


chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
where one chemical entity (a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
or molecular fragment) interposes itself into an existing bond of typically a second chemical entity ''e.g.'':
:
The term only refers to the result of the reaction and does not suggest a mechanism. Insertion reactions are observed in organic, inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemis ...
, and organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
chemistry. In cases where a metal-ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
bond in a coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
is involved, these reactions are typically organometallic in nature and involve a bond between a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
and a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
or hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. It is usually reserved for the case where the coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
and oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
of the metal remain unchanged. When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination.
There are two common insertion geometries— 1,1 and 1,2 (pictured above). Additionally, the inserting molecule can act either as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
or as an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
to the metal complex. These behaviors will be discussed in more detail for CO, nucleophilic behavior, and SO2, electrophilic behavior.
Organic chemistry
Homologation reaction
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant u ...
s like the Kowalski ester homologation The Kowalski ester homologation is a chemical reaction for the homologation of esters.
This reaction was designed as a safer alternative to the Arndt–Eistert synthesis, avoiding the need for diazomethane. The Kowalski reaction is named after ...
provide simple examples of insertion process in organic synthesis. In the Arndt-Eistert reaction, a methylene unit is inserted into the carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
-carbon bond of carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
to form the next acid in the homologous series
In organic chemistry, a homologous series is a sequence of compounds with the same functional group and similar chemical properties in which the members of the series can be branched or unbranched, or differ by molecular formula of and molecu ...
. ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and exper ...
'' provides the example of ''t''-BOC protected (''S'')-phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
(2-amino-3-phenylpropanoic acid) being reacted sequentially with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
, ethyl chloroformate, and diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almos ...
to produce the α-diazoketone, which is then reacted with silver trifluoroacetate / triethylamine in aqueous solution to generate the ''t''-BOC protected form of (''S'')-3-amino-4-phenylbutanoic acid.
: Mechanistically, the α-diazoketone undergoes a
Mechanistically, the α-diazoketone undergoes a Wolff rearrangement
The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate prod ...
to form a ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound eth ...
in a 1,2-rearrangement A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over l ...
. Consequently, the methylene group α- to the carboxyl group in the product is the methylene group from the diazomethane reagent. The 1,2-rearrangement has been shown to conserve the stereochemistry of the chiral centre as the product formed from ''t''-BOC protected (''S'')-phenylalanine retains the (''S'') stereochemistry with a reported enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a s ...
of at least 99%.
A related transformation is the Nierenstein reaction in which a diazomethane methylene group is inserted into the carbon-chlorine bond of an acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
to generate an α-chloro ketone. An example, published in 1924, illustrates the reaction in a substituted benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It i ...
system:
: Perhaps surprisingly, α-bromoacetophenone is the minor product when this reaction is carried out with
Perhaps surprisingly, α-bromoacetophenone is the minor product when this reaction is carried out with benzoyl bromide
In organic chemistry, benzoyl (, ) is the functional group with the formula C6H5CO-. It can be viewed as benzaldehyde missing one hydrogen.
The term "benzoyl" should not be confused with benzyl, which has the formula C6H5CH2. The benzoyl gr ...
, a dimeric dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
being the major product. Organic azides also provide an example of an insertion reaction in organic synthesis and, like the above examples, the transformations proceed with loss of nitrogen gas
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at sevent ...
. When tosyl azide reacts with norbornadiene, a ring expansion reaction takes place in which a nitrogen atom is inserted into a carbon-carbon bond α- to the bridge head:
: The
The Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones ...
is another example of a ring expanding reaction in which a heteroatom is inserted into a carbon-carbon bond. The most important application of this reaction is the conversion of cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohex ...
to its oxime, which is then rearranged under acidic conditions to provide ε-caprolactam
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is us ...
, the feedstock for the manufacture of Nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the compa ...
. Annual production of caprolactam exceeds 2 billion kilograms.
:Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" may ...
s undergo both intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
and intramolecular insertion reactions. Cyclopentene moieties can be generated from sufficiently long-chain ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s by reaction with trimethylsilyldiazomethane, (CH3)3Si–CHN2:
:reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these com ...
s that behave similarly to carbenes. One example is the chloroalkyllithium carbenoid reagent prepared ''in situ'' from a sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
and ''t''-BuLi which inserts into the carbon-boron bond of a pinacol boronic ester:
:
Organometallic chemistry
Many reactions in organometallic chemistry involve insertion of one ligand (L) into a metal-hydride or metal-alkyl/aryl bond. Generally it is the hydride, alkyl, or aryl group that migrates onto L, which is often CO, an alkene, or alkyne.Carbonylations
The insertion of carbon monoxide and alkenes into metal-carbon bonds is a widely exploited reaction with major industrial applications. Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but
Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
are especially important in determining the stereochemistry and regiochemistry of the reactions. The reverse reaction, the de-insertion of CO and alkenes, are of fundamental significance in many catalytic cycles as well.
Widely employed applications of migratory insertion of carbonyl groups are hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
and the carbonylative production of acetic acid. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the rhodium-based Monsanto acetic acid process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemic ...
, but this process has been superseded by the iridium-based Cativa process. By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
: The Cativa process
The Cativa process catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, material ...
, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
with (1) involves the formal insertion of the iridium(I) centre into the carbon-iodine bond, whereas step (3) to (4) is an example of migratory insertion of carbon monoxide into the iridium-carbon bond. The active catalyst species is regenerated by the reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and ...
of acetyl iodide
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is p ...
from (4), a de-insertion reaction.
Olefin insertion
The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler-Natta catalysis, the commercial route of polyethylene and polypropylene. This technology mainly involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions. Related technologies include theShell Higher Olefin Process
The Shell higher olefin process (SHOP) is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell.''Industrial Organic Chemistry'', Klaus Weisserm ...
which produces detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
precursors. the olefin can be coordinated to the metal before insertion. Depending on the ligand density of the metal, ligand dissociation may be necessary to provide a coordination site for the olefin.

Other insertion reactions in coordination chemistry
Many electrophilic oxides insert into metal carbon bonds; these includesulfur dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ...
, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, and nitric oxide. These reactions have limited practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complexes, the insertion of SO2 has been examined in particular detail.
More insertion reactions in organic chemistry
Electropositive metals such as sodium, potassium, magnesium, zinc, etc. can insert into alkyl halides, breaking the carbon-halide bond ( halide could be chlorine, bromine, iodine ) and forming a carbon-metal bond. This reaction happens via a SET mechanism ( single-electron-transfer mechanism ). If magnesium reacts with an alkyl halide, it forms a Grignard reagent, or if lithium reacts, anorganolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
is formed. Thus, this type of insertion reactions has important applications in chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mo ...
.

References
{{DEFAULTSORT:Insertion Reaction Organometallic chemistry