|
Nuclear Localization
A nuclear localization signal ''or'' sequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus. Types Classical These types of NLSs can be further classified as either monopartite or bipartite. The major structural differences between the two are that the two basic amino acid clusters in bipartite NLSs are separated by a relatively short spacer sequence (hence bipartite - 2 parts), while monopartite NLSs are not. The first NLS to be discovered was the sequence PKKKRKV in the SV40 Large T-antigen (a monopartite NLS). The NLS of nucleoplasmin, KR AATKKAGQAKKK, is the prototype of the ubiquitous bipartite signal: two cluster ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenopus
''Xenopus'' () (Gk., ξενος, ''xenos''=strange, πους, ''pous''=foot, commonly known as the clawed frog) is a genus of highly aquatic frogs native to sub-Saharan Africa. Twenty species are currently described within it. The two best-known species of this genus are ''Xenopus laevis'' and ''Xenopus tropicalis'', which are commonly studied as model organisms for developmental biology, cell biology, toxicology, neuroscience and for modelling human disease and birth defects. The genus is also known for its polyploidy, with some species having up to 12 sets of chromosomes. Characteristics ''Xenopus laevis'' is a rather inactive creature. It is incredibly hardy and can live up to 15 years. At times the ponds that ''Xenopus laevis'' is found in dry up, compelling it, in the dry season, to burrow into the mud, leaving a tunnel for air. It may lie dormant for up to a year. If the pond dries up in the rainy season, ''Xenopus laevis'' may migrate long distances to another pond, main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine Nucleotide Exchange Factor
Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase. Function Guanine nucleotide exchange factors (GEFs) are proteins or protein domains involved in the activation of small GTPases. Small GTPases act as molecular switches in intracellular signaling pathways and have many downstream targets. The most well-known GTPases comprise the Ras superfamily and are involved in essential cell processes such as cell differentiation and proliferation, cytoskeletal organization, vesicle trafficking, and nuclear transport. GTPases are active when bound to GTP and inactive when bound to GDP, allowing their activity to be regulated by GEFs and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase-activating Protein
GTPase-activating proteins or GTPase-accelerating proteins (GAPs) are a family of regulatory proteins whose members can bind to activated G proteins and stimulate their GTPase activity, with the result of terminating the signaling event. GAPs are also known as RGS protein, or RGS proteins,Kimple, A.J. "Structural Determinants of G-protein α Subunit Selectivity by Regulator of G-protein Signaling 2 (RGS2)". ''The Journal of Biological Chemistry''. 284 (2009): 19402-19411. and these proteins are crucial in controlling the activity of G proteins. Regulation of G proteins is important because these proteins are involved in a variety of important cellular processes. The large G proteins, for example, are involved in transduction of signaling from the G protein-coupled receptor for a variety of signaling processes like hormonal signaling, and small G proteins are involved in processes like cellular trafficking and cell cycling. GAP's role in this function is to turn the G protein's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karyopherin
Karyopherins are proteins involved in transporting molecules between the cytoplasm and the nucleus of a eukaryotic cell. The inside of the nucleus is called the karyoplasm (or nucleoplasm). Generally, karyopherin-mediated transport occurs through nuclear pores which acts as a gateway into and out of the nucleus. Most proteins require karyopherins to traverse the nuclear pore. Karyopherins can act as ''importins'' (i.e. helping proteins get into the nucleus) or ''exportins'' (i.e. helping proteins get out of the nucleus). They belong to the nuclear pore complex family in the transporter classification database (TCDB). Energy for transport is derived from the Ran gradient. Upon stress, several karyopherins stop shuttling between the nucleus and the cytoplasm and are sequestered in stress granules, cytoplasmic aggregates of ribonucleoprotein complexes. Importin beta Importin beta is a variety of karyopherin that facilitates the transport of cargo proteins into the nucleus. First, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
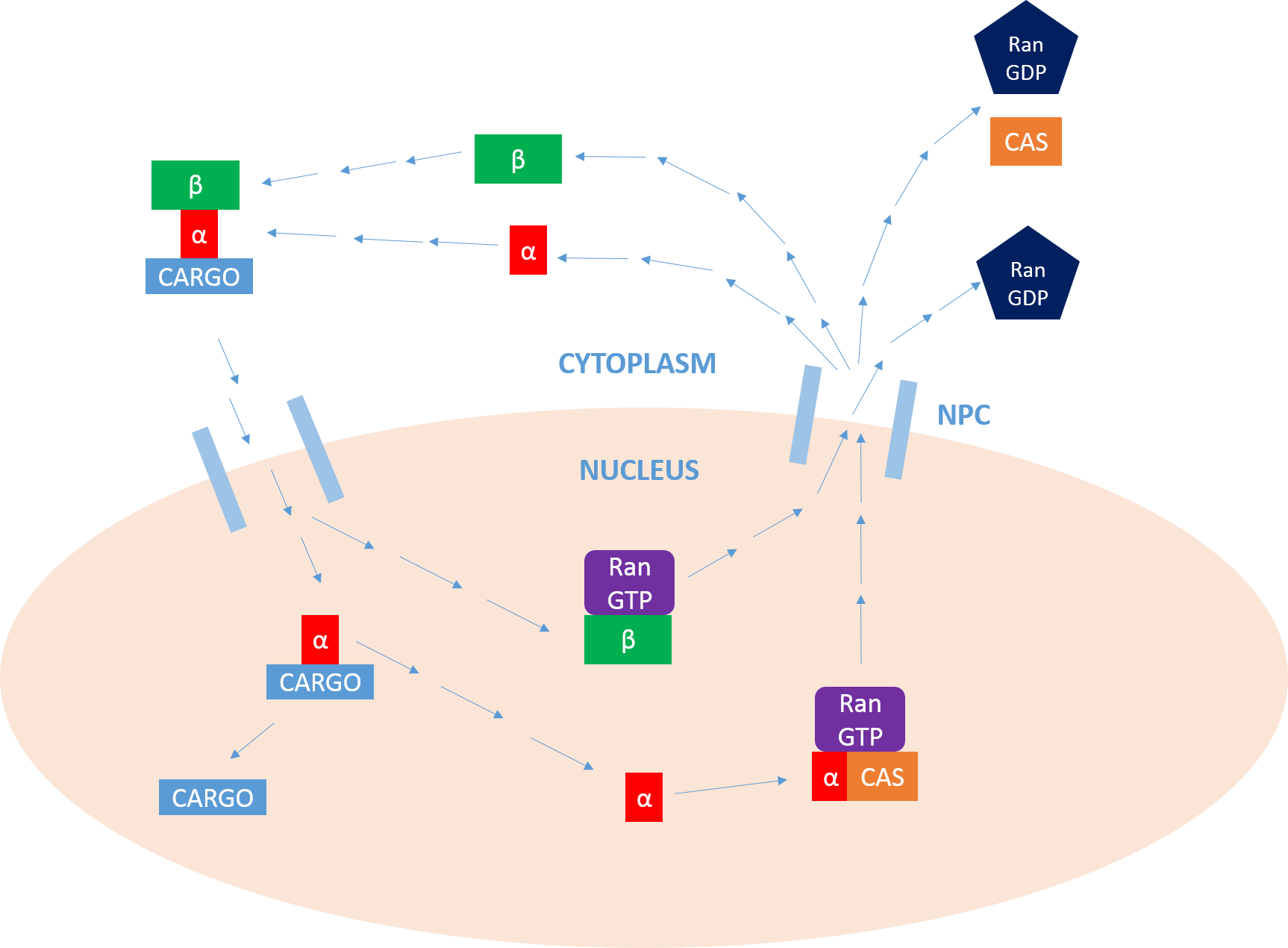
Importin
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS). Importin has two subunits, importin α and importin β. Members of the importin-β family can bind and transport cargo by themselves, or can form heterodimers with importin-α. As part of a heterodimer, importin-β mediates interactions with the pore complex, while importin-α acts as an adaptor protein to bind the nuclear localization signal (NLS) on the cargo. The NLS-Importin α-Importin β trimer dissociates after binding to Ran GTP inside the nucleus, with the two importin proteins being recycled to the cytoplasm for further use. Discovery Importin can exist as either a heterodimer of importin-α/β or as a monomer of Importin-β. Importin-α was first isolated in 1994 by a group includinEnno Hartmann based at the Max Delbrück Center for Molecular Medicine. The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Pore Complex
A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes (NPCs) in the nuclear envelope of a vertebrate cell, but this number varies depending on cell type and the stage in the life cycle. The human nuclear pore complex (hNPC) is a 110 megadalton (MDa) structure. The proteins that make up the nuclear pore complex are known as nucleoporins; each NPC contains at least 456 individual protein molecules and is composed of 34 distinct nucleoporin proteins. About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ran (biology)
Ran (RAs-related Nuclear protein) also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily. Ran is a small G protein that is essential for the translocation of RNA and proteins through the nuclear pore complex. The Ran protein has also been implicated in the control of DNA synthesis and cell cycle progression, as mutations in Ran have been found to disrupt DNA synthesis. Function Ran cycle Ran exists in the cell in two nucleotide-bound forms: GDP-bound and GTP-bound. RanGDP is converted into RanGTP through the action of RCC1, the nucleotide exchange factor for Ran. RCC1 is also known as RanGEF (Ran Guanine nucleotide Exchange Factor). Ran's intrinsic GTPase-activity is activated through interaction with Ran GTPase activating protein (RanGAP), facilit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the generalized recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Journal Of Cell Biology
The ''Journal of Cell Biology'' is a peer-reviewed scientific journal published by Rockefeller University Press. History In the early 1950s, a small group of biologists began to explore intracellular anatomy using the emerging technology of electron microscopy. Many of these researchers were at The Rockefeller Institute of Medicine, the predecessor of The Rockefeller University. As their work progressed to publication, they were disappointed with the limited quality of halftone image reproduction in the printed journals of the time, and frustrated by the narrow editorial policies of existing journals regarding their image-based results. In 1954, the Director of the Rockefeller Institute, Detlev Bronk, convened a luncheon to discuss the creation of a new journal as a venue for publication of this type of work. The first issue of ''The Journal of Biophysical and Biochemical Cytology'' was published less than a year later on January 25, 1955. A subscription cost $15 per year. The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structure (journal)
''Structure'' is a monthly peer-reviewed scientific journal established in September 1993 Wayne Hendrickson, Carl-Ivar Brändén, and Alan R. Fersht. It focuses on structural biology, studies of macromolecular structure, and related issues. In early 1999, the journal merged with ''Folding & Design'' and the name changed to ''Structure with Folding & Design''. In 2001, the journal reverted to ''Structure''. The journal is published by Cell Press and Christopher D. Lima and Andrej Sali served as editors-in-chief An editor-in-chief (EIC), also known as lead editor or chief editor, is a publication's editorial leader who has final responsibility for its operations and policies. The highest-ranking editor of a publication may also be titled editor, managing ... from 2003 to October 2021. The journal is now edited by an in-house team at Cell Press. External links * Biochemistry journals Cell Press academic journals Publications established in 1993 Monthly journals English ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |