|
Novolak
Novolaks (sometimes: novolacs) are low molecular weight polymers derived from phenols and formaldehyde. They are related to Bakelite, which is more highly crosslinked. The term comes from Swedish "lack" for lacquer and Latin "novo" for new, since these materials were envisioned to replace natural lacquers such as copal resin. Typically novolaks are prepared by the condensation of phenol or a mixture of p- and m-cresol with formaldehyde (as formalin). The reaction is acid catalyzed. Oxalic acid is often used because it can be subsequently removed by thermal decomposition. Novolaks have a degree of polymerization of approximately 20-40. The branching density, determined by the processing conditions, m- vs p-cresol ratio, as well as CH2O/cresol ratio is typically around 15%. Novolaks are especially important in microelectronics where they are used as photoresist materials. They are also used as tackifier Tackifiers are chemical compounds used in formulating adhesives to incr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy
Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, composites, use in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
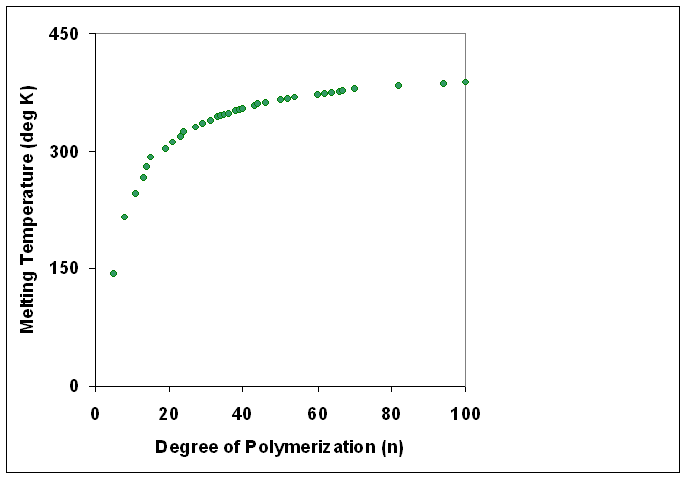
Degree Of Polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by DP_n\equiv X_n=\frac, where Mn is the number-average molecular weight and M0 is the molecular weight of the monomer unit. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units. Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27 For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO—, so that a chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Resins
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not. Synthetic resins are of several classes. Some are manufactured by esterification of organic compounds. Some are thermosetting plastics in which the term "resin" is loosely applied to the reactant(s), the product, or both. "Resin" may be applied to one of two monomers in a copolymer, the other being called a "hardener", as in epoxy resins. For thermosetting plastics that require only one monomer, the monomer compound is the "resin". For example, liquid methyl methacrylate is often called the "resin" or "casting resin" while in the liquid state, before it polymerizes and "sets". After setting, the resulting poly(methyl methacrylate) (PMMA) is often rename ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastics
Plastics are a wide range of synthetic polymers, synthetic or semi-synthetic materials that use polymers as a main ingredient. Their Plasticity (physics), plasticity makes it possible for plastics to be Injection moulding, moulded, Extrusion, extruded or Compression molding, pressed into solid objects of various shapes. This adaptability, plus a wide range of other properties, such as being lightweight, durable, flexible, and inexpensive to produce, has led to its widespread use. Plastics typically are made through human industrial systems. Most modern plastics are derived from petrochemical, fossil fuel-based chemicals like natural gas or petroleum; however, recent industrial methods use variants made from renewable materials, such as corn or cotton derivatives. 9.2 billion tonnes of plastic are estimated to have been made between 1950 and 2017. More than half this plastic has been produced since 2004. In 2020, 400 million tonnes of plastic were produced. If global trends on pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Formaldehyde Resins
Phenol (also called carbolic acid) is an aromaticity, aromatic organic compound with the molecular chemical formula, formula . It is a white crystalline solid that is volatility (chemistry), volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor (chemistry), precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivative (chemistry), derivatives are essential for production of polycarbonates, epoxy, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably solubility, soluble in water, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tackifier
Tackifiers are chemical compounds used in formulating adhesives to increase the tack, the stickiness of the surface of the adhesive. They are usually low-molecular weight compounds with high glass transition temperature. At low strain rate, they provide higher stress compliance, and become stiffer at higher strain rates. Tackifiers tend to have low molecular weight, and glass transition and softening temperature above room temperature, providing them with suitable viscoelastic properties. Tackifiers frequently represent most of both weight percentage and cost of hot melt adhesives and pressure-sensitive adhesives. In hot melt adhesives they can comprise up to about 40% of total mass. Tackifiers are usually resins (e.g. rosins and their derivates, terpenes and modified terpenes, aliphatic, cycloaliphatic and aromatic resins (C5 aliphatic resins, C9 aromatic resins, and C5/C9 aliphatic/aromatic resins), hydrogenated hydrocarbon resins, and their mixtures, terpene-phenol resins (TPR, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronic industry. The process begins by coating a substrate with a light-sensitive organic material. A patterned mask is then applied to the surface to block light, so that only unmasked regions of the material will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive material is degraded by light and the developer will dissolve away the regions that were exposed to light, leaving behind a coating where the mask was placed. In the case of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the developer will dissolve away only the regions that were not exposed to light, leaving behind a coating in areas w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make Calcium oxide, quick lime, which is an industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus ''Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cresol
Cresols (also hydroxytoluene or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also categorized as methylphenols. Cresols commonly occur as either solids or liquids because their melting points are generally close to room temperature. Like other types of phenols, they are slowly oxidized by exposure to air, and the resulting impurities often give the samples a yellow to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name "cresol" is an adduct of phenol and their traditional source, creosote. Structure and production In its chemical structure, a molecule of cresol has a methyl group substituted onto the ring of phenol. There are three forms (isomers) of cresol: ''ortho''-cresol ( ''o''-cresol), ''meta''-cresol ( ''m''-cresol), and ''para''-cresol ( ''p''-cresol). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |