Degree of polymerization on:
[Wikipedia]
[Google]
[Amazon]
The degree of polymerization, or DP, is the number of monomeric units in a
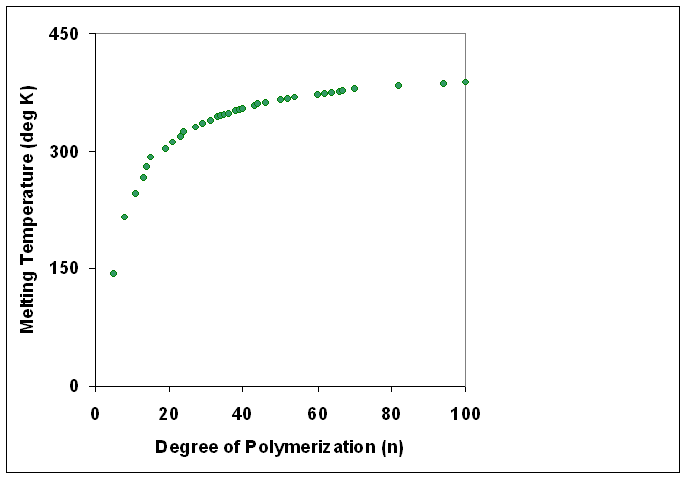 Polymers with identical composition but different molecular weights may exhibit different physical properties. In general, increasing degree of polymerization correlates with higher melting temperature Flory, P.J. and Vrij, A. J. Am. Chem. Soc.; 1963; 85(22) pp3548-3553 Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons., doi=10.1021/ja00905a004, url=http://pubs.acs.org/doi/abs/10.1021/ja00905a004 and higher mechanical strength.
Polymers with identical composition but different molecular weights may exhibit different physical properties. In general, increasing degree of polymerization correlates with higher melting temperature Flory, P.J. and Vrij, A. J. Am. Chem. Soc.; 1963; 85(22) pp3548-3553 Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons., doi=10.1021/ja00905a004, url=http://pubs.acs.org/doi/abs/10.1021/ja00905a004 and higher mechanical strength.
macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
or polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
or oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by ,
where Mn is the number-average molecular weight The molar mass distribution (or molecular weight distribution) describes the relationship between the number of Mole (unit), moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chain ...
and M0 is the molecular weight of the monomer unit. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units.
Some authors, however, define DP as the number of repeat unit
In polymer chemistry, a repeat unit or repeating unit (or mer) is a part of a polymer whose repetition would produce the complete polymer chain (except for the end-groups) by linking the repeat units together successively along the chain, like the ...
s, where for copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
s the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27 For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO—, so that a chain of 1000 monomeric units corresponds to 500 repeat units. The degree of polymerization or chain length is then 1000 by the first (IUPAC) definition, but 500 by the second.
Step-growth and chain-growth polymerization
Instep-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring a ...
, in order to achieve a high degree of polymerization (and hence molecular weight), ''X''n, a high fractional monomer conversion, ''p'', is required, according to Carothers' equation
In step-growth polymerization, the Carothers equation (or Carothers' equation) gives the degree of polymerization, , for a given fractional monomer conversion, .
There are several versions of this equation, proposed by Wallace Carothers, who inve ...
For example, a monomer conversion of ''p'' = 99% would be required to achieve ''X''n = 100.
For chain-growth free radical polymerization, however, Carothers' equation does not apply. Instead long chains are formed from the beginning of the reaction. Long reaction times increase the polymer yield, but have little effect on the average molecular weight. The degree of polymerization is related to the kinetic chain length In polymer chemistry the kinetic chain length of a polymer, ''ν'', is the average number of units called monomers added to a growing chain during chain-growth polymerization. During this process, a polymer chain is formed when monomers are bonded t ...
, which is the average number of monomer molecules polymerized per chain initiated. However it often differs from the kinetic chain length for several reasons:
* chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination
In polymer chemistry, ...
may occur wholly or partly by recombination of two chain radicals, which doubles the degree of polymerization
* chain transfer
Chain transfer is a polymerization Chemical reaction, reaction by which the activity of a growing polymer chain is transferred to another molecule.
:P• + XR' → PX + R'•
Chain transfer reactions reduce the average molecular weight of the fi ...
to monomer starts a new macromolecule for the same kinetic chain (of reaction steps), corresponding to a decrease of the degree of polymerization
* chain transfer to solvent or to another solute (a ''modifier'' or ''regulator'' also decreases the degree of polymerization
Correlation with physical properties
 Polymers with identical composition but different molecular weights may exhibit different physical properties. In general, increasing degree of polymerization correlates with higher melting temperature Flory, P.J. and Vrij, A. J. Am. Chem. Soc.; 1963; 85(22) pp3548-3553 Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons., doi=10.1021/ja00905a004, url=http://pubs.acs.org/doi/abs/10.1021/ja00905a004 and higher mechanical strength.
Polymers with identical composition but different molecular weights may exhibit different physical properties. In general, increasing degree of polymerization correlates with higher melting temperature Flory, P.J. and Vrij, A. J. Am. Chem. Soc.; 1963; 85(22) pp3548-3553 Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons., doi=10.1021/ja00905a004, url=http://pubs.acs.org/doi/abs/10.1021/ja00905a004 and higher mechanical strength.
Number-average and weight-average
Synthetic polymers invariably consist of a mixture of macromolecular species with different degrees of polymerization and therefore of different molecular weights. There are different types of average polymer molecular weight, which can be measured in different experiments. The two most important are the number average (Xn) and the weight average (Xw). The ''number-average degree of polymerization'' is aweighted mean
The weighted arithmetic mean is similar to an ordinary arithmetic mean (the most common type of average), except that instead of each of the data points contributing equally to the final average, some data points contribute more than others. The ...
of the degrees of polymerization of polymer species, weighted by the ''mole fractions'' (or the number of molecules) of the species. It is typically determined by measurements of the osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
of the polymer.
The ''weight-average degree of polymerization'' is a weighted mean of the degrees of polymerization, weighted by the ''weight fractions'' (or the overall weight of the molecules) of the species. It is typically determined by measurements of Rayleigh light scattering by the polymer.
See also
*Anhydroglucose unit The anhydroglucose unit (AGU) refers to a single sugar molecule in a polymer. Each AGU is reduced to its functional groups, 3 hydroxyl groups per AGU.
Carbohydrate AGU:
Cellulose AGU:
{, class="wikitable"
!Polymer
!AGU{{Cite book, title=Cellulo ...
References
{{reflist Polymer chemistry