|
Norbornadiene Synthesis
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize (isomers would be anti-Bredt alkenes). Norbornadiene is also a useful dienophile in Diels-Alder reactions. Synthesis Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene : Reactions Quadricyclane, a valence isomer, can be obtained from norbornadiene by a photochemical reaction when assisted by a sensitizer such as acetophenone: : The norbornadiene-quadricyclane couple is of potential interest for solar energy storage when controlled release of the strain energy stored in quadricyclane back to norbornadiene is made possible. Norbornadiene is reactive in cycloaddition reactions. Norbornadiene is also the starting material f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances. Production Acetophenone is formed as a byproduct of the cumene process, the industrial route for the synthesis of phenol and acetone. In the Hock rearrangement of isopropylbenzene hydroperoxide, migration of a methyl group rather than the phenyl group gives acetophenone and methanol as a result of an alternate rearrangement of the intermediate: :C6H5C(CH3)2O2H -> C6H5C(O)CH3 + CH3OH The cumene process is conducted on such a large scale that even the small amount of acetophenone by-product can be recovered in commercially useful quantities. Acetophenone is also generated from ethylbenzene hydroperoxide. Ethylbenzene hydroperoxide is primarily converted to 1-phenylethanol (α-methylbenzyl alcohol) in the process with a small amount of by-product acetophenone. Acetophenone is recovered or hydrogena ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal donation from Alan R. Katritzky and Linde Katritzky. ''Arkivoc'' is the primary publication of Arkat USA. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.165, ranking it 37th out of 57 journals in the category "Chemistry, Organic". Abstracting and Indexing According to the Journal Citation Reports, the journal has a 2018 impact factor of 1.253. The journal is indexed in Web of Science: Science Citation Index Expanded. References External link ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(Norbornadiene)molybdenum Tetracarbonyl
(Norbornadiene)molybdenum tetracarbonyl is the organomolybdenum compound with the formula (C7H9)Mo(CO)4. Structurally, the compound consists of the norbornadiene bonded to a Mo(CO)4 fragment. The compound is a yellow, volatile solid. It is prepared by thermal or photochemical substitution of molybdenum hexacarbonyl. The compound was originally examined as a potential antiknock agent An antiknock agent is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, when used in high compr .... (Norbornadiene)molybdenum tetracarbonyl is a precursor to other derivatives of the type L2Mo(CO)4. This conversion exploits the lability of the diene ligand: :(C7H9)Mo(CO)4 + 2 L → C7H9 + L2Mo(CO)4 References {{DEFAULTSORT:Norbornadiene)molybdenum tetracarbonyl Molybdenum(0) compounds Carbonyl complexes Octahedral compounds Diene co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctadiene Rhodium Chloride Dimer
Cyclooctadiene rhodium chloride dimer is the organorhodium compound with the formula Rh2Cl2(C8H12)2, commonly abbreviated [RhCl(COD)]2 or Rh2Cl2(COD)2. This yellow-orange, air-stable compound is a widely used precursor to homogeneous catalysis, homogeneous catalysts.Giordano, G.; Crabtree, R. H. “Di-μ-chloro-bis(η4-1,5-cyclooctadiene)dirhodium(I)” Inorganic Syntheses, 1990, volume 28, pages 88-90. Preparation and reactions The synthesis of [RhCl(COD)]2 involves heating a solution of hydrated rhodium trichloride with 1,5-cyclooctadiene in aqueous ethanol in the presence of sodium carbonate: :2 RhCl3·3H2O + 2 COD + 2 CH3CH2OH + 2 Na2CO3 → [RhCl(COD)]2 + 2 acetaldehyde, CH3CHO + 8 H2O + 2 CO2 + 4 NaCl [RhCl(COD)]2 is principally used as a source of the electrophile "[Rh(COD)]+." :[RhCl(COD)]2 + L → [LRh(COD)]+Cl− (where L = PR3, alkene, etc. and = 2 or 3) In this way, Chirality (chemistry), chiral phosphines can be attached to Rh. The resulting chiral complexes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecules (journal)
''Molecules'' is a peer-reviewed open access scientific journal that focuses on all aspects of chemistry and materials science. It was established in March 1996 and is published monthly by MDPI. From 1997 to 2001, ''Molbank'' was published as a section of the journal, before splitting into its own journal. The editor-in-chief is Farid Chemat. ''Molecules'' was initially published by Springer-Verlag. In December 1996, Shu-Kun Lin resigned as editor and relaunched the journal with Molecular Diversity Preservation International (MDPI). Springer initially sued over naming rights, but eventually dropped the suit. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,6-di-2-pyridyl-1,2,4,5-tetrazine
Tetrazine is a compound that consists of a six-membered aromatic ring containing four nitrogen atoms with the molecular formula C2 H2 N4. The name ''tetrazine'' is used in the nomenclature of derivatives of this compound. Three core-ring isomers exist: 1,2,3,4-tetrazines, 1,2,3,5-tetrazines, and 1,2,4,5-tetrazines, also known as v-tetrazines, as-tetrazines and s-tetrazines respectively. 1,2,3,4-Tetrazines 1,2,3,4-Tetrazines are often isolated fused to an aromatic ring system and are stabilized as the dioxide derivatives. 1,2,4,5-Tetrazine 1,2,4,5-Tetrazines are very well known and myriad 3,6-disubstituted 1,2,4,5-tetrazines are known. These materials are of use in the area of energetic chemistry. The compound 3,6-di-2-pyridyl-1,2,4,5-tetrazine' has two pyridine substituents and is of importance as a reagent in Diels-Alder reactions. It reacts with norbornadiene in a sequence of one DA reaction and two retro-DA reactions to cyclopentadiene and a pyridazine with exchange of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
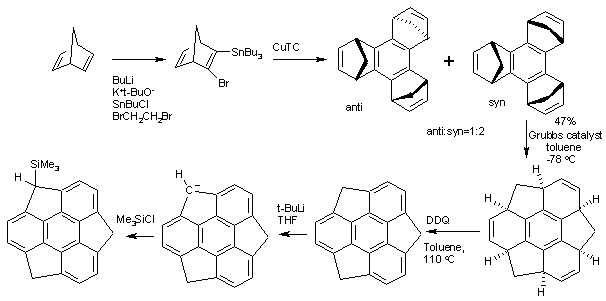
Sumanene
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. ''Suman'' means "sunflower" in both Hindi and Sanskrit. The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions. Organic synthesis The structure of Sumanene can be inferred from oxidation of 1,5,9-trimethyltriphenylene but the first practical synthesis starts from norbornadiene. Norbornadiene is converted into a stannane by action of ''n''-butyllithium, dibromoethane and tributyltinchloride. An Ullmann reaction of this stannane with CuTC affords the benzene core. The methylene bridges (--) created in this conversion then migrate in a tandem ring-opening metathesis and ring-closing metathesis by the Grubbs' catalyst. The final structure is obtained by oxidation by DDQ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and experiments reported in an article must be successfully repeated in the laboratory of a member of the editorial board as a check for reproducibility prior to publication. The journal is published by Organic Syntheses, Inc., a non-profit corporation. An annual print version is published by John Wiley & Sons on behalf of Organic Syntheses, Inc. History Prior to World War I, work on synthetic organic chemistry in the United States had been quite limited, and most of the reagents used in laboratories had to be imported from Europe. When export stoppages and trade embargoes cut off this source, Clarence Derick, a professor of chemistry at University of Illinois at Urbana-Champaign, began an effort to synthesize these needed chemicals in industr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamantane
Diamantane (also called congressane) is an organic compound that is a member of the diamondoids. These are cage hydrocarbons with structures similar to a subunit of the diamond lattice. It is a colorless solid that has been a topic of research since its discovery in oil and separation from deep natural gas condensates. Diamondoids such as diamantane exhibit unusual properties, including low surface energies, high densities, high hydrophobicities, and resistance to oxidation. Occurrence and history Diamantane occurs naturally in crude petroleum. It is currently assumed that adamantanes and diamantanes were formed via the catalytic rearrangements of polycyclic naphthenic hydrocarbons. Although present in only trace concentrations in typical oils, due to their great thermodynamic stability, diamondoids such as diamantane are naturally concentrated by catagenesis, becoming important constituents of some natural gas condensates including those from the Norphlet Formation, U.S. Gulf of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |