|
Nitrostarch
Nitrostarch is the common name of a secondary explosive similar to nitrocellulose. Much like starch, it is made up of two components, nitrated amylose and nitrated amylopectin. Nitrated amylopectin generally has a greater solubility than amylose; however, it is less stable than nitrated amylose. The solubility, detonation velocity, and impact sensitivity depends heavily on the level of nitration. Synthesis Nitrostarch is made by dissolving starch in red fuming nitric acid. It is then precipitated by adding the solution to concentrated sulfuric acid. C6H12O6 + 4NHO3 4C6H6N4O13 + 4H2O Nitrostarch can be stabilized by refluxing it in ethanol to drive off the left over nitric acid. History Nitrostarch was first discovered by French chemist and pharmacist Henri Braconnot. In World War I, it was used as a filler in hand grenades A grenade is an explosive weapon typically thrown by hand (also called hand grenade), but can also refer to a shell (explosive projectile) sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the Interna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
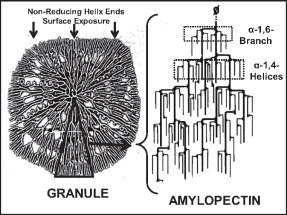
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α- glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolyzation of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Dissolved amylopectin starch has a lower tendency of retrogradation (a partial recrystalli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was List of wars and anthropogenic disasters by death toll, one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fighting occurring throughout Europe, the Middle East, Africa, the Pacific Ocean, Pacific, and parts of Asia. An estimated 9 million soldiers were killed in combat, plus another 23 million wounded, while 5 million civilians died as a result of military action, hunger, and disease. Millions more died in Genocides in history (World War I through World War II), genocides within the Ottoman Empire and in the Spanish flu, 1918 influenza pandemic, which was exacerbated by the movement of combatants during the war. Prior to 1914, the European great powers were divided between the Triple Entente (comprising French Third Republic, France, Russia, and British Empire, Britain) and the Triple A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henri Braconnot
Henri Braconnot (29 May 1780 – 13 January 1855) was a French chemist and pharmacist. He was born in Commercy, his father being a counsel at the local parliament. At the death of his father, in 1787, Henri began his instruction in an elementary school in Commercy and then with private teachers. At 13, he was placed as apprentice in a pharmacy in Nancy where he learned and practiced pharmacy, chemistry, and botany. At 15, he left Nancy for a military service in an hospital in Strasbourg. In 1801-1802, he lived in Paris where he learnt in various schools, Museum, school of medicine among others, and followed the lectures of Antoine François, comte de Fourcroy, Jean-Baptiste Lamarck and Étienne Geoffroy Saint-Hilaire. He carried out some chemical investigations on the composition of a fossil horn which were published later (J Chim Phys 1806). From 1802 to his death, he lived in Nancy where he was named in 1807 as director of the botanical garden and member of the scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacist
A pharmacist, also known as a chemist (Commonwealth English) or a druggist (North American and, archaically, Commonwealth English), is a healthcare professional who prepares, controls and distributes medicines and provides advice and instructions on the correct and safe use of medicines to achieve maximum benefit, minimal side effects and to avoid drug interactions. They also serve as primary care providers in the community. Pharmacists undergo university or graduate-level education to understand the biochemical mechanisms and actions of drugs, drug uses, therapeutic roles, side effects, potential drug interactions, and monitoring parameters. This is mated to anatomy, physiology, and pathophysiology. Pharmacists interpret and communicate this specialized knowledge to patients, physicians, and other health care providers. Among other licensing requirements, different countries require pharmacists to hold either a Bachelor of Pharmacy, Master of Pharmacy, or Doctor of Pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms. Chemists carefully measure substance proportions, chemical reaction rates, and other chemical properties. In Commonwealth English, pharmacists are often called chemists. Chemists use their knowledge to learn the composition and properties of unfamiliar substances, as well as to reproduce and synthesize large quantities of useful naturally occurring substances and create new artificial substances and useful processes. Chemists may specialize in any number of subdisciplines of chemistry. Materials scientists and metallurgists share much of the same education and skills with chemists. The work of chemists is often related to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
French People
The French people (french: Français) are an ethnic group and nation primarily located in Western Europe that share a common French culture, history, and language, identified with the country of France. The French people, especially the native speakers of langues d'oïl from northern and central France, are primarily the descendants of Gauls (including the Belgae) and Romans (or Gallo-Romans, western European Celtic and Italic peoples), as well as Germanic peoples such as the Franks, the Visigoths, the Suebi and the Burgundians who settled in Gaul from east of the Rhine after the fall of the Roman Empire, as well as various later waves of lower-level irregular migration that have continued to the present day. The Norse also settled in Normandy in the 10th century and contributed significantly to the ancestry of the Normans. Furthermore, regional ethnic minorities also exist within France that have distinct lineages, languages and cultures such as Bretons in B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time. Reflux in industrial distillation The term ''reflux'' is very widely used in industries that utilize large-scale distillation columns and fractionators such as petroleum refineries, petrochemical and chemical plants, and natural gas processing plants. In that context, reflux refers to the portion of the overhead liquid product from a distillation column or fractionator that is returned to the upper part of the column as shown in the schematic diagram of a typical industrial distillation column. Inside the column, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation column. The more reflux provided for a given num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Fuming Nitric Acid
Red fuming nitric acid (RFNA) is a storable oxidizer used as a rocket propellant. It consists of 84% nitric acid (), 13% dinitrogen tetroxide and 1–2% water. The color of red fuming nitric acid is due to the dinitrogen tetroxide, which breaks down partially to form nitrogen dioxide. The nitrogen dioxide dissolves until the liquid is saturated, and produces toxic fumes with a suffocating odor. RFNA increases the flammability of combustible materials and is highly exothermic when reacting with water. It is usually used with an inhibitor (with various, sometimes secret, substances, including hydrogen fluoride; any such combination is called ''inhibited RFNA'', ''IRFNA'') because nitric acid attacks most container materials. Hydrogen fluoride for instance will passivate the container metal with a thin layer of metal fluoride, making it nearly impervious to the nitric acid. It can also be a component of a monopropellant; with substances like amine nitrates dissolved in it, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
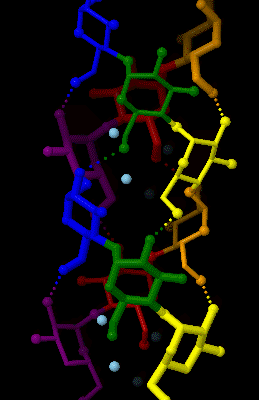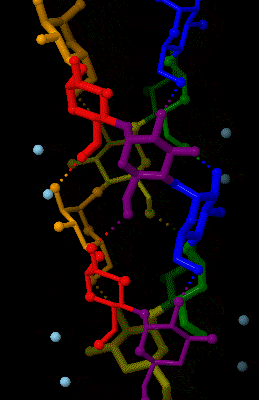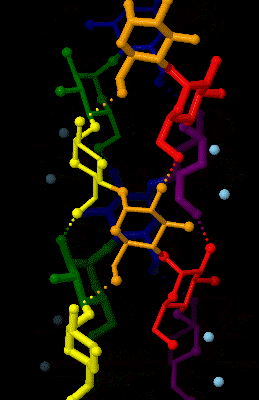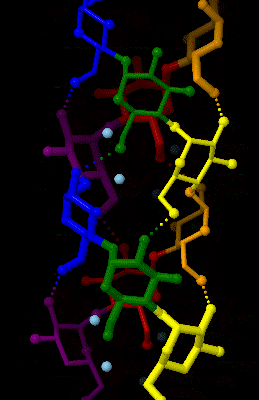
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–30%. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |