|
Nitrosonium Tetrafluoroborate
Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent. NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, Osup>+, and a tetrafluoroborate anion, F4sup>−. Reactions The dominant property of NOBF4 is the oxidizing power and electrophilic character of the nitrosonium cation. It forms colored charge transfer complexes with hexamethylbenzene and with 18-crown-6. The latter, a deep yellow species, provides a means to dissolve NOBF4 in dichloromethane. Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type II(CH3CN)''x''F4sub>2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas: : M + 2NOBF4 + ''x''CH3CN → (CH3CN)''x''BF4)2 + 2NO With ferrocene Ferrocene is an organometallic compound with the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchlorate anion, , which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. As an anion in inorganic and organic chemistry The popularization of has led to decreased use of in the laboratory as a weakly coordinating anion. With organic compounds, especially amine derivatives, forms potentially explosive derivatives. Disadvantages to include its slight sensitivity to hydrolysis and decomposition via loss of a fluoride ligand, whereas does not suffer from these problems. Safety considerations, however, overshadow this inconvenience. With a formula weight of 86.8, BF is also conveni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrocenium Tetrafluoroborate
Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe(C5H5)2]BF4. This salt is composed of the cation [Fe(C5H5)2]+ and the tetrafluoroborate anion (). The related hexafluorophosphate is also a popular reagent with similar properties. The cation is often abbreviated Fc+ or Cyclopentadienide, Cp2Fe+. The salt is deep blue in color and paramagnetic. Ferrocenium salts are sometimes used as one-electron oxidizing agents, and the reduced product, ferrocene, is inert and readily separated from ionic products. The ferrocene–ferrocenium couple is often used as a reference in electrochemistry. The standard potential of ferrocene-ferrocenium is 0.400 V vs. the normal hydrogen electrode (NHE) and is often assumed to be invariant between different solvents. Preparation Commercially available, this compound may be prepared by oxidizing ferrocene typically with ferric salts followed by addition of fluoroboric acid. A variety of other oxidants work well also, su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation . The rapid growth of organometallic chemistry is often attributed to the excitement arising from the discovery of ferrocene and its many analogues, such as metallocenes. History Discovery Ferrocene was discovered by accident thrice. The first known synthesis may have been made in the late 1940s by unknown researchers at Union Carbide, who tried to pass hot cyclopentadiene vapor through an iron pipe. The vapor reacted with the pipe wall, creating a "yellow sludg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-crown-6
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is in cyclohexane and in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen. Synthesis This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation: It can be also prepared by the oligomerization of ethylene oxide: :(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O It can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Transfer Complex
In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, i.e., one has at least partial negative charge and the partner has partial positive charge, referred to respectively as the electron acceptor and electron donor. In some cases, the degree of charge transfer is "complete", such that the CT complex can be classified as a salt. In other cases, the charge-transfer association is weak, and the interaction can be disrupted easily by polar solvents. Examples Electron donor-acceptor complexes A number of organic compounds form charge-transfer complex, which are often described as electron-donor-acceptor complexes (EDA complexes). Typical acceptors are nitrobenzenes or tetracyanoethylene. The strength of their interaction with electron donors correlates with the ionization potentials of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosonium
The nitrosonium ion is , in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: , (nitrosylsulfuric acid, more descriptively written ) and . The and salts are slightly soluble in acetonitrile . NOBF4 can be purified by sublimation at 200–250 °C and . is isoelectronic with CO, and . It arises via protonation of nitrous acid: :HONO + H+ NO+ + H2O Chemical properties Hydrolysis reacts readily with water to form nitrous acid: : For this reason, nitrosonium compounds must be protected from water or even moist air. With base, the reaction generates nitrite: : As a diazotizing agent reacts with aryl amines, , to give diazonium salts, . The resulting diazonium group is easily displaced (unlike the amino group) by a variety of nucleophiles. As an oxidizing agent , e.g. as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
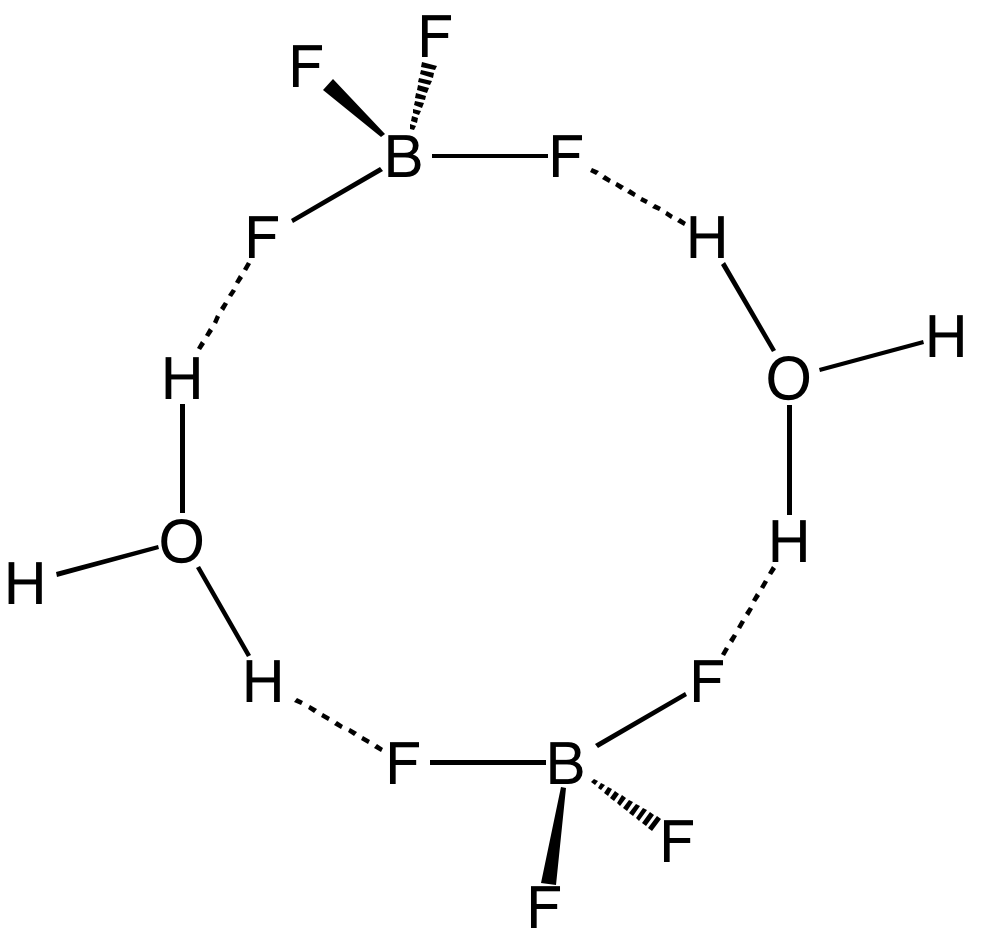
Fluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |