|
Nitecapone
Nitecapone (INN; OR-462) is a drug which acts as a selective inhibitor of the enzyme catechol ''O''-methyl transferase (COMT). It was patented as an antiparkinson medication but was never marketed. See also * Catechol-O-methyltransferase inhibitor A catechol-''O''-methyltransferase (COMT) inhibitor is a drug that inhibits the enzyme catechol-''O''-methyltransferase. This enzyme methylates catecholamines such as dopamine, norepinephrine and epinephrine. It also methylates levodopa. COMT in ... References Catechol-O-methyltransferase inhibitors Catechols Diketones Nitrophenols {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol-O-methyl Transferase
Catechol-''O''-methyltransferase (COMT; ) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-''O''-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957. Function Catechol-''O''-methyltransferase is involved in the inactivation of the catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine). The enzyme introduces a methyl group to the catecholamine, which is donated by S-adenosyl methionine (SAM). Any compound having a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
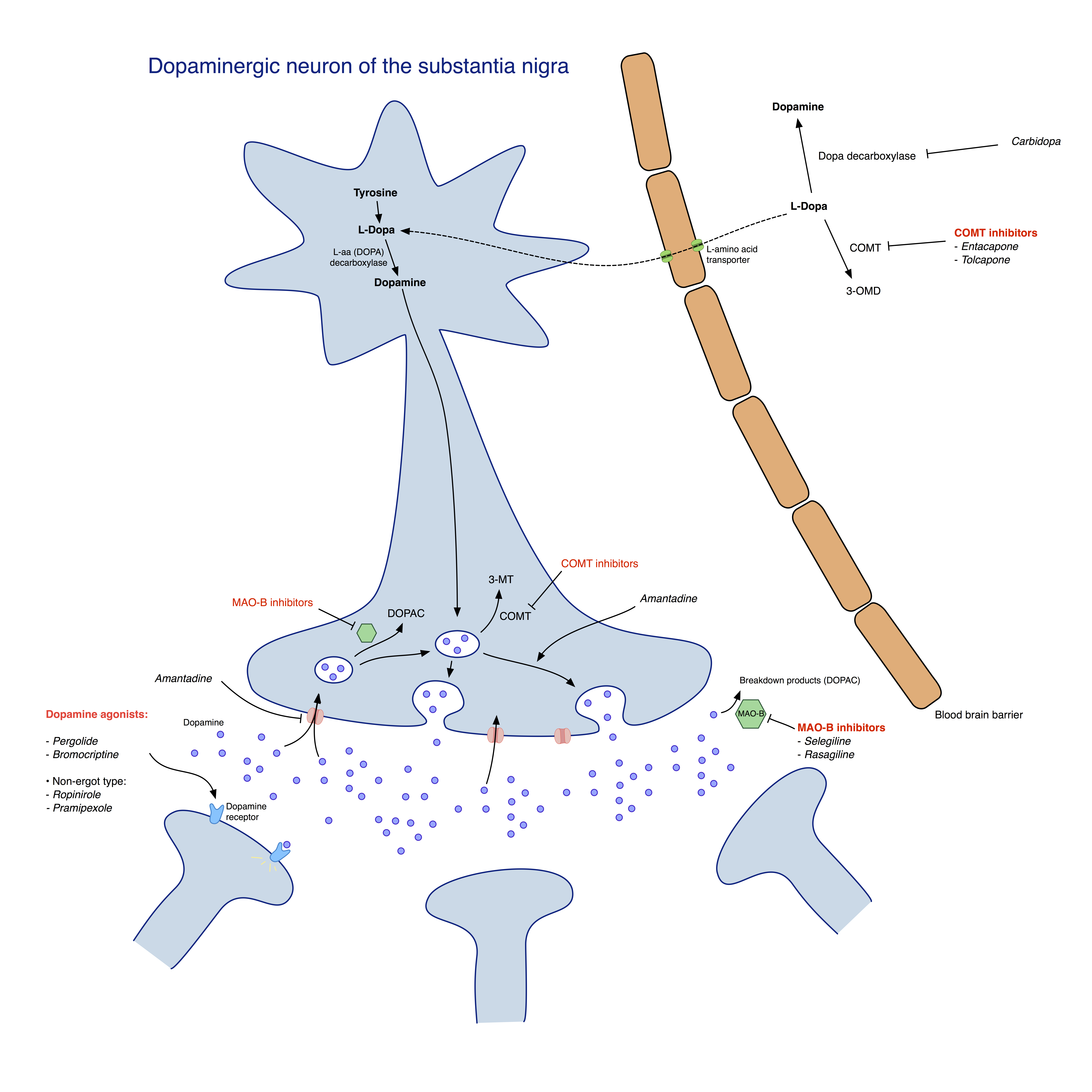
Catechol-O-methyltransferase Inhibitor
A catechol-''O''-methyltransferase (COMT) inhibitor is a drug that inhibits the enzyme catechol-''O''-methyltransferase. This enzyme methylates catecholamines such as dopamine, norepinephrine and epinephrine. It also methylates levodopa. COMT inhibitors are indicated for the treatment of Parkinson's disease in combination with levodopa and an aromatic L-amino acid decarboxylase inhibitor (e.g. carbidopa or benserazide). The therapeutic benefit of using a COMT inhibitor is based on its ability to prevent the methylation of levodopa to 3-''O''-methyldopa, thus increasing the bioavailability of levodopa. COMT inhibitors significantly decrease off time in people with Parkinson's disease also taking carbidopa/levodopa. List of COMT inhibitors * entacapone (Comtan, Comtess, Stalevo) *nebicapone * nitecapone * opicapone (Ongentys) * tolcapone (Tasmar) Entacapone and opicapone are peripheral inhibitors, unable to cross the blood-brain barrier. Tolcapone is able to cross the blood-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol-O-methyltransferase Inhibitors
Catechol-''O''-methyltransferase (COMT; ) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-''O''-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957. Function Catechol-''O''-methyltransferase is involved in the inactivation of the catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine). The enzyme introduces a methyl group to the catecholamine, which is donated by S-adenosyl methionine (SAM). Any compound having a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Selectivity
Binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes. Selectivity coefficient The concept of selectivity is used to quantify the extent to which one chemical substance, A, binds each of two other chemical substances, B and C. The simplest case is where the complexes formed have 1:1 stoichiometry. Then, the two interactions may be characterized by equilibrium constants ''K''AB and ''K''AC.The constant used here are ''association'' constants. ''Dissociation'' constants are used in some contexts. A dissociation constant is the reciprocal of an association constant. : + B AB; \mathit K_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Management Of Parkinson's Disease
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms. While many medications treat Parkinson's, none actually reverses the effects of the disease. Furthermore, the gold-standard treatment varies with the disease state. People with Parkinson's, therefore, often must take a variety of medications to manage the disease's symptoms. Several medications currently in development seek to better address motor fluctuations and nonmotor symptoms of PD. However, none is yet on the market with specific approval to treat Parkinson's. Medication The main families of drugs useful for treating motor symptoms are levodopa, dopamine agonists, and MAO-B inhibitors. The most commonly used treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechols
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' (''Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketones
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condenses re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |