|
Nickel Sulphate
Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the chemical formula, formula NiSO4(H2O)6. This highly soluble blue green coloured salt (chemistry), salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005. It is mainly used for electroplating of nickel. In 2005–2006, nickel sulfate was the top allergen in patch tests (19.0%).Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60. Structures At least seven sulfate salts of nickel(II) are known. These salts differ in terms of their water of hydration, hydration or crystal habit. The common tetragonal hexahydrate crystallizes from aqueous solution between 30.7 and 53.8 °C. Below these temperatures, a heptahydrate crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
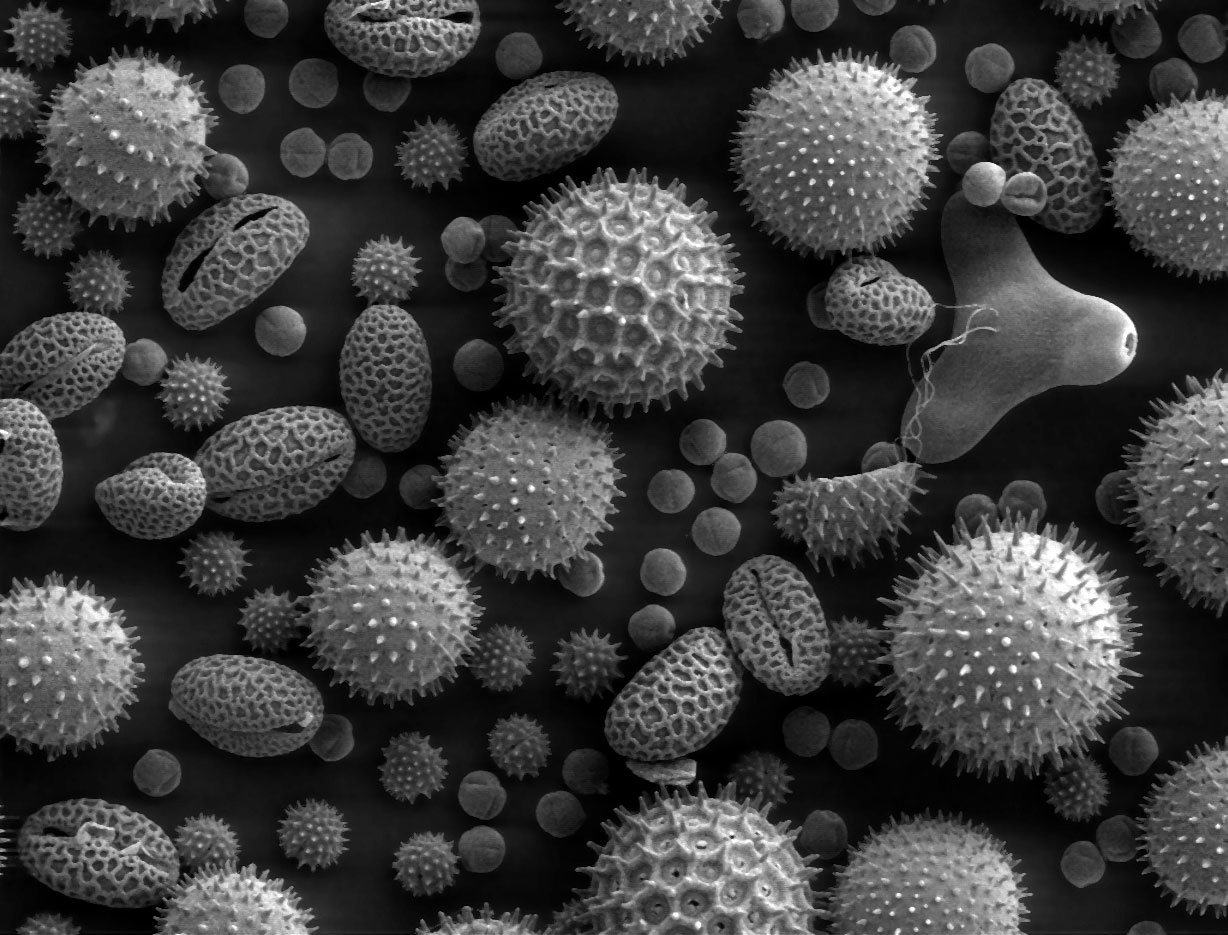
Allergen
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies. In technical terms, an allergen is an antigen that is capable of stimulating a type-I hypersensitivity reaction in atopic individuals through immunoglobulin E (IgE) responses. Most humans mount significant Immunoglobulin E responses only as a defense against parasitic infections. However, some individuals may respond to many common environmental antigens. This hereditary predisposition is called atopy. In atopic individuals, non-parasitic antigens stimulate inappropriate IgE production, leading to type I hypersensitivity. Sensitivities vary widely from one person (or from one animal) to another. A very broad range of substances can be allergens to sensitive individuals. Types of allergens Allergens can be found in a variety of sources, such as dust mite e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhistidine-tag
A polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (''His'') residues, often at the N- or C-terminus of the protein. It is also known as hexa histidine-tag, 6xHis-tag, His6 tag, by the US trademarked name HIS TAG (US Trademark serial number 74242707), and most commonly as His-Tag. The tag was invented by Roche, although the use of histidines and its vectors are distributed by Qiagen. Various purification kits for histidine-tagged proteins are available from Qiagen, Sigma-Aldrich Corporation, Sigma, Thermo Scientific, GE Healthcare, Macherey-NagelCube Biotech Clontech, Bio-Radand others. MK(HQ)6 may be used for enhanced expression in ''E. coli'' and tag removal. The total number of histidine residues may vary in the tag from as low as two, to as high as 10 or more His residues. N- or C-terminal His-tags may also be followed or preceded, respectively, by a suitable amino acid sequence that facilitates removal of the polyhistidine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohr's Salt
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2(H2O)6. Containing two different cations, Fe2+ and NH4+, it is classified as a double salt of Iron(II) sulfate, ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the metal aquo complex, aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite. Structure This compound is a member of a group of double sulfates called Schönites or Tutton's salts. Tutton's salts form monoclinic crystals and have formula M2N(SO4)2.6H2O (M = various monocations). With regards to the bonding, crystals consist of octahedral molecular geometry, octahedra [Fe(H2O)6]2+ centers, which are hydrogen bonded to sulfate and ammonium. Mohr's salt is named after the German chemist Karl Friedric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Sulfate
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur. Uses The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil, while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate, which elevates transportation costs.Karl-Heinz Zapp "Ammonium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2012, Wiley-VCH, Weinheim. It is also used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Carbonate
Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, the most important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel. Structure and reactions NiCO3 adopts a structure like calcite, consisting of nickel in an octahedral coordination geometry. Nickel carbonates are hydrolyzed upon contact with aqueous acids to give solutions containing the ion i(H2O)6sup>2+, liberating water and carbon dioxide in the process. Calcining (heating to drive off CO2 and water) of these carbonates gives nickel oxide: :NiCO3 → NiO + CO2 The nature of the resulting oxide depends on the nat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)