|
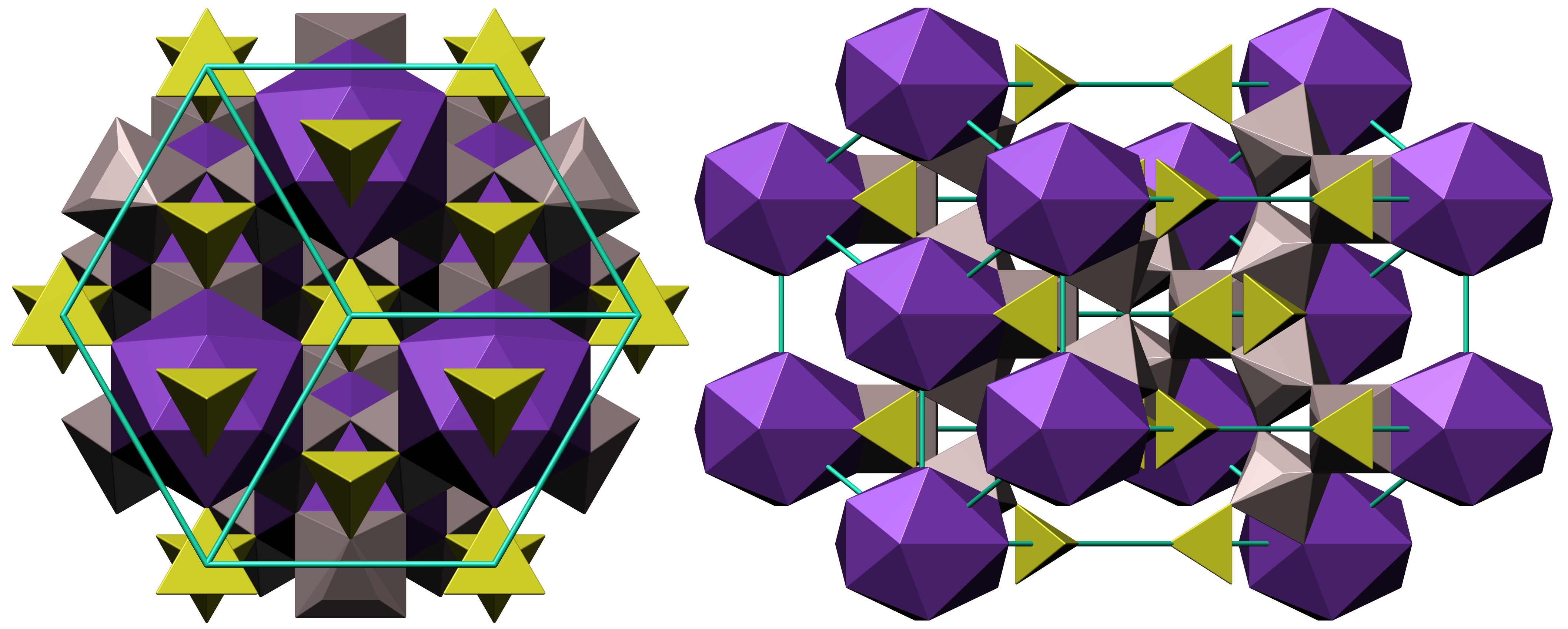
Natroalunite
Alunite is a hydroxylated aluminium potassium sulfate mineral, formula K Al3( S O4)2(O H)6. It was first observed in the 15th century at Tolfa, near Rome, where it was mined for the manufacture of alum. First called ''aluminilite'' by J.C. Delamétherie in 1797, this name was contracted by François Beudant three decades later to alunite. Alunite crystals morphologically are rhombohedron, rhombohedra with interfacial angles of 90° 50', causing them to resemble cubes. Crystal symmetry is trigonal. Minute glistening crystals have also been found loose in cavities in altered rhyolite. Alunite varies in color from white to yellow gray. The hardness on the Mohs scale is 4 and the specific gravity is between 2.6 and 2.8. It is insoluble in water or weak acids, but soluble in sulfuric acid. Sodium can substitute for potassium in the mineral, and when the sodium content is high, is called natroalunite. Alunite is an analog of jarosite, where aluminium replaces Fe3+. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 ** Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite Cu3SO4(OH)4 **Brochantite Cu4SO4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates ** Barite BaSO4 **Celestite SrSO4 ** Anglesite PbSO4 ** Anhydrite CaSO4 ** Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O ** Chalcanthite CuSO4·5H2O ** Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O ** Hexahydrite MgSO4·6H2O ** Epsomite MgSO4·7H2O ** Meridianiite MgSO4·11H2O ** Melanterite FeSO4·7H2O ** Antlerite Cu3SO4(OH)4 **Broc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhombohedron
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a three-dimensional figure with six faces which are rhombi. It is a special case of a parallelepiped where all edges are the same length. It can be used to define the rhombohedral lattice system, a honeycomb with rhombohedral cells. A cube is a special case of a rhombohedron with all sides square. In general a ''rhombohedron'' can have up to three types of rhombic faces in congruent opposite pairs, ''C''''i'' symmetry, order 2. Four points forming non-adjacent vertices of a rhombohedron necessarily form the four vertices of an orthocentric tetrahedron, and all orthocentric tetrahedra can be formed in this way. Rhombohedral lattice system The rhombohedral lattice system has rhombohedral cells, with 6 congruent rhombic faces forming a trigonal trapezohedron: : Special cases by symmetry * Cube: with Oh symmetry, order 48. All faces are squares. * Trigonal trapezohedron (also c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volcanic Rocks
Volcanic rock (often shortened to volcanics in scientific contexts) is a rock formed from lava erupted from a volcano. In other words, it differs from other igneous rock by being of volcanic origin. Like all rock types, the concept of volcanic rock is artificial, and in nature volcanic rocks grade into hypabyssal and metamorphic rocks and constitute an important element of some sediments and sedimentary rocks. For these reasons, in geology, volcanics and shallow hypabyssal rocks are not always treated as distinct. In the context of Precambrian shield geology, the term "volcanic" is often applied to what are strictly metavolcanic rocks. Volcanic rocks and sediment that form from magma erupted into the air are called "volcaniclastics," and these are technically sedimentary rocks. Volcanic rocks are among the most common rock types on Earth's surface, particularly in the oceans. On land, they are very common at plate boundaries and in flood basalt provinces. It has been estimate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trachyte
Trachyte () is an extrusive igneous rock composed mostly of alkali feldspar. It is usually light-colored and aphanitic (fine-grained), with minor amounts of mafic minerals, and is formed by the rapid cooling of lava enriched with silica and alkali metals. It is the volcanic equivalent of syenite. Trachyte is common wherever alkali magma is erupted, including in late stages of ocean island volcanismMacDonald 1983, pp. 51-52 and in continental rift valleys, above mantle plumes,Philpotts and Ague 2009, pp. 390-394 and in areas of back-arc extension. Trachyte has also been found in Gale crater on Mars. Trachyte has been used as decorative building stone and was extensively used as dimension stone in the Roman Empire and the Republic of Venice. Chemical composition Trachyte has a silica content of 60 to 65% and an alkali oxide content of over 7%. This gives it less SiO2 than rhyolite and more (Na2O plus K2O) than dacite. These chemical differences are consistent with the posi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jarosite
Jarosite is a basic hydrous sulfate of potassium and ferric iron (Fe-III) with a chemical formula of KFe3(SO4)2(OH)6. This sulfate mineral is formed in ore deposits by the oxidation of iron sulfides. Jarosite is often produced as a byproduct during the purification and refining of zinc and is also commonly associated with acid mine drainage and acid sulfate soil environments. Physical properties Jarosite has a trigonal crystal structure and is brittle, with basal cleavage, a hardness of 2.5-3.5, and a specific gravity of 3.15-3.26. It is translucent to opaque with a vitreous to dull luster, and is colored dark yellow to yellowish-brown. It can sometimes be confused with limonite or goethite with which it commonly occurs in the gossan (oxidized cap over an ore body). Jarosite is an iron analogue of the potassium aluminium sulfate, alunite. Solid solution series The alunite supergroup includes the alunite, jarosite, beudantite, crandallite and florencite subgroups. The alu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alunite Crystal Structure
Alunite is a hydroxylated aluminium potassium sulfate mineral, formula K Al3( S O4)2(O H)6. It was first observed in the 15th century at Tolfa, near Rome, where it was mined for the manufacture of alum. First called ''aluminilite'' by J.C. Delamétherie in 1797, this name was contracted by François Beudant three decades later to alunite. Alunite crystals morphologically are rhombohedron, rhombohedra with interfacial angles of 90° 50', causing them to resemble cubes. Crystal symmetry is trigonal. Minute glistening crystals have also been found loose in cavities in altered rhyolite. Alunite varies in color from white to yellow gray. The hardness on the Mohs scale is 4 and the specific gravity is between 2.6 and 2.8. It is insoluble in water or weak acids, but soluble in sulfuric acid. Sodium can substitute for potassium in the mineral, and when the sodium content is high, is called natroalunite. Alunite is an analog of jarosite, where aluminium replaces Fe3+. Alunite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alunite Basic Hydrous Aluminum Potassium Sulfate Marysvale Piute County Utah 2324
Alunite is a hydroxylated aluminium potassium sulfate mineral, formula K Al3( S O4)2(O H)6. It was first observed in the 15th century at Tolfa, near Rome, where it was mined for the manufacture of alum. First called ''aluminilite'' by J.C. Delamétherie in 1797, this name was contracted by François Beudant three decades later to alunite. Alunite crystals morphologically are rhombohedron, rhombohedra with interfacial angles of 90° 50', causing them to resemble cubes. Crystal symmetry is trigonal. Minute glistening crystals have also been found loose in cavities in altered rhyolite. Alunite varies in color from white to yellow gray. The hardness on the Mohs scale is 4 and the specific gravity is between 2.6 and 2.8. It is insoluble in water or weak acids, but soluble in sulfuric acid. Sodium can substitute for potassium in the mineral, and when the sodium content is high, is called natroalunite. Alunite is an analog of jarosite, where aluminium replaces Fe3+. Alunite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |