|
Nanometric
The nanoscopic scale (or nanoscale) usually refers to structures with a length scale applicable to nanotechnology, usually cited as 1–100 nanometers (nm). A nanometer is a billionth of a meter. The nanoscopic scale is (roughly speaking) a lower bound to the mesoscopic scale for most solids. For technical purposes, the nanoscopic scale is the size at which fluctuations in the averaged properties (due to the motion and behavior of individual particles) begin to have a significant effect (often a few percent) on the behavior of a system, and must be taken into account in its analysis. The nanoscopic scale is sometimes marked as the point where the properties of a material change; above this point, the properties of a material are caused by 'bulk' or 'volume' effects, namely which atoms are present, how they are bonded, and in what ratios. Below this point, the properties of a material change, and while the type of atoms present and their relative orientations are still importan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Translation
In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesize proteins after the process of transcription of DNA to RNA in the cell's nucleus. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later folds into an active protein and performs its functions in the cell. The ribosome facilitates decoding by inducing the binding of complementary tRNA anticodon sequences to mRNA codons. The tRNAs carry specific amino acids that are chained together into a polypeptide as the mRNA passes through and is "read" by the ribosome. Translation proceeds in three phases: # Initiation: The ribosome assembles around the target mRNA. The first tRNA is attached at the start codon. # Elongation: The last tRNA validated by the small ribosomal subunit (''accommodati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Machines Of Life
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexible Linker
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like aggregates, or flexible linkers in large multi-domain proteins. They are sometimes considered as a separate class of proteins along with globular, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins and their discovery has disproved the idea that three-dimensional structures of proteins must be fixed to accomplish their biological functions. For example, IDPs have been identified to participate in weak multivalent interactions that are highly cooperative and dynamic, lending them importance in DNA regulation and in cell signaling. Many IDPs can also adopt a fixed three-dimensional structure after bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flagella
A flagellum (; ) is a hairlike appendage that protrudes from certain plant and animal sperm cells, and from a wide range of microorganisms to provide motility. Many protists with flagella are termed as flagellates. A microorganism may have from one to many flagella. A gram-negative bacterium ''Helicobacter pylori'' for example uses its multiple flagella to propel itself through the mucus lining to reach the stomach epithelium, where it may cause a gastric ulcer to develop. In some bacteria the flagellum can also function as a sensory organelle, being sensitive to wetness outside the cell. Across the three domains of Bacteria, Archaea, and Eukaryota the flagellum has a different structure, protein composition, and mechanism of propulsion but shares the same function of providing motility. The Latin word means " whip" to describe its lash-like swimming motion. The flagellum in archaea is called the archaellum to note its difference from the bacterial flagellum. Eukaryoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cilia
The cilium, plural cilia (), is a membrane-bound organelle found on most types of eukaryotic cell, and certain microorganisms known as ciliates. Cilia are absent in bacteria and archaea. The cilium has the shape of a slender threadlike projection that extends from the surface of the much larger cell body. Eukaryotic flagella found on sperm cells and many protozoans have a similar structure to motile cilia that enables swimming through liquids; they are longer than cilia and have a different undulating motion. There are two major classes of cilia: ''motile'' and ''non-motile'' cilia, each with a subtype, giving four types in all. A cell will typically have one primary cilium or many motile cilia. The structure of the cilium core called the axoneme determines the cilium class. Most motile cilia have a central pair of single microtubules surrounded by nine pairs of double microtubules called a 9+2 axoneme. Most non-motile cilia have a 9+0 axoneme that lacks the central pair of mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
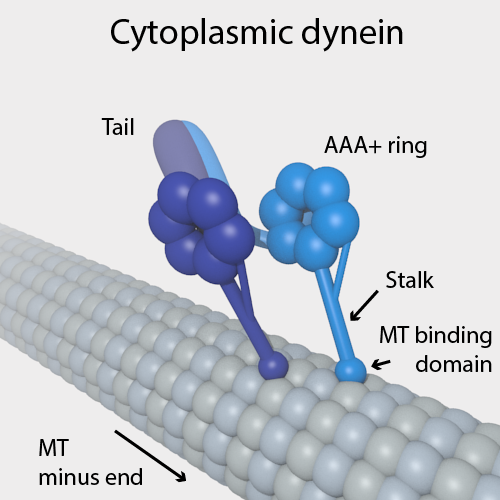
Dynein
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, DYNLL2, DYNLRB1, DYNLRB2, DYNLT1, DYNLT3 * axo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, organell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Nucleus
The cell nucleus (pl. nuclei; from Latin or , meaning ''kernel'' or ''seed'') is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support. The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long stands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinesin
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases, a type of enzyme). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport. Discovery Kinesins were discovered in 1985, based on their motility in cytoplasm extruded from the giant axon of the squid. They turned out as MT-based anterograde intracellular trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscle tissue, and are often known as muscle fibers. The muscle tissue of a skeletal muscle is striated – having a striped appearance due to the arrangement of the sarcomeres. Skeletal muscles are voluntary muscles under the control of the somatic nervous system. The other types of muscle are cardiac muscle which is also striated and smooth muscle which is non-striated; both of these types of muscle tissue are classified as involuntary, or, under the control of the autonomic nervous system. A skeletal muscle contains multiple fascicles – bundles of muscle fibers. Each individual fiber, and each muscle is surrounded by a type of connective tissue layer of fascia. Muscle fibers are formed from the fusion of developmental myoblasts in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motor Proteins
Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump. Cellular functions Motor proteins are the driving force behind most active transport of proteins and vesicles in the cytoplasm. Kinesins and cytoplasmic dyneins play essential roles in intracellular transport such as axonal transport and in the formation of the spindle apparatus and the separation of the chromosomes during mitosis and meiosis. Axonemal dynein, found in cilia and flagella, is crucial to cell motility, for example in spermatozoa, and fluid transport, for example in trachea. The muscle protein myosin "motors" the contraction of muscle fibers in animals. Diseases associated with motor protein defects The importance of motor proteins in cells becomes evident when they fail to fulfill their function. For example, kinesin deficiencies have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |