|
Naloxol
Naloxol is an opioid antagonist closely related to naloxone. It exists in two isomeric forms, α-naloxol and β-naloxol. α-naloxol is a human metabolite of naloxone. Synthetically, α-naloxol can be prepared from naloxone by reduction of the ketone group, and β-naloxol can be prepared from α-naloxol by a Mitsunobu reaction. Naloxol can be said to be the oxymorphol analogue of naloxone. See also * Naloxegol Naloxegol (INN; PEGylated naloxol; trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was a ... References 4,5-Epoxymorphinans Human drug metabolites Mu-opioid receptor antagonists Allyl compounds {{Analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naloxegol
Naloxegol (INN; PEGylated naloxol; trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia. The most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting and headache. Patients often describe the above side effects to be similar to an instant withdrawal state brought on quickly rather than the 24 hours it may take to occur naturally. As a pure opioid antagonist Naloxegol has no potential for abuse. Naloxegol was previously a Schedule II drug in the United States because of its chemical similarity to opium alkaloids. It was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
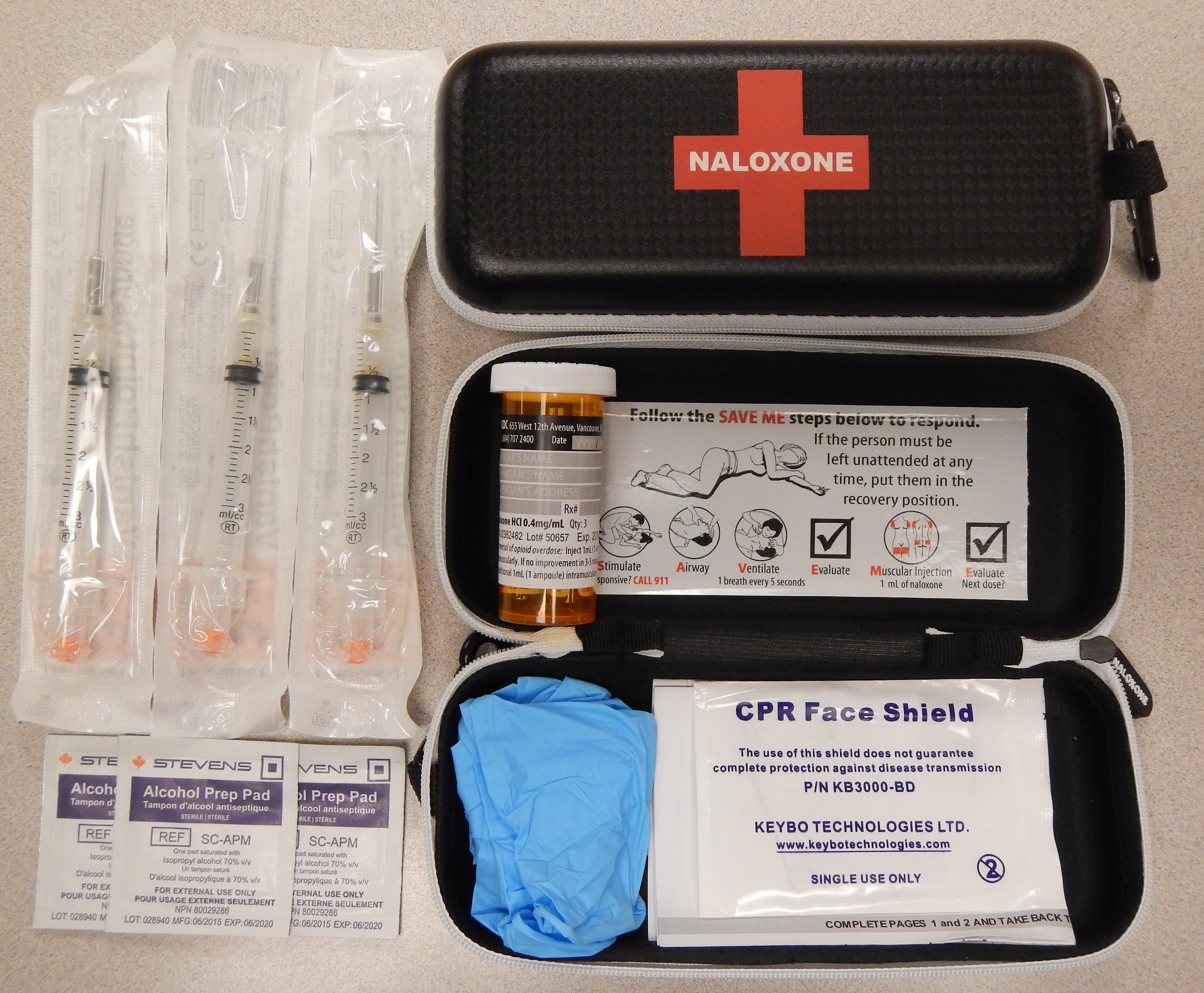
Naloxone
Naloxone, sold under the brand names Narcan (4 mg) and Kloxxado (8 mg) among others, is a medication used to reverse or reduce the effects of opioids. It is commonly used to counter decreased breathing in opioid overdose. Effects begin within two minutes when given intravenously, and within five minutes when injected into a muscle. The medicine can also be administered by spraying it into a person's nose. Naloxone commonly blocks the effects of opioids for 30 to 90 minutes. Multiple doses may be required, as the duration of action of some opioids is greater than that of naloxone. Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal, including restlessness, agitation, nausea, vomiting, a fast heart rate, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Antagonist
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors. Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This effectively blocks the receptor, preventing the body from responding to opioids and endorphins. Some opioid antagonists are not pure antagonists but do produce some weak opioid partial agonist effects, and can produce analgesic effects when administered in high doses to opioid-naive individuals. Examples of such compounds include nalorphine and levallorphan. However, the analgesic effects from these specific drugs are limited and tend to be accompanied by dysphoria, most likely due to additional agonist action at the κ-opioid receptor. As they induce opioid withdrawal effects in people who are taking, or have recently used, opioid full agonists, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary metabolite is directly involved in normal "growth", development, and reproduction. Ethylene exemplifies a primary metabolite produced large-scale by industrial microbiology. A secondary metabolite is not directly involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some antibiotics use primary metabolites as precursors, such as actinomycin, which is created from the primary metabolite tryptophan. Some sugars are metabolites, such as fructose or glucose, which are both p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitsunobu Reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). Typical protocol is to add the phosphine and azodicarboxylate together at −10 °C, typically in THF or toluene, until a white precipitate forms. This white, cloudy suspension is the ylide. Then a solution of the nucleophile and alcohol are added together and the reaction can be, and in many cases is, heated to reflux. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxymorphol
Oxymorphol is oxymorphone which has been hydrogenated at the 6-position and consists of a mixture of 4,5α-Epoxy-17-methylmorphinan-3,6β,14-triol and 4,5α-Epoxy-17-methylmorphinan-3,6α,14-triol (hydromorphinol). . It is produced by the human body as an active metabolite of oxymorphone and some bacteria as an intermediate in turning morphine into hydromorphone Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is an opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mou .... It can also be manufactured and is the subject of patents by drug companies looking for new semi-synthetic analgesics and cough suppressants. A derivative of oxymorphol, 8-hydroxy-6-α-oxymorphol, was discovered in the first decade of this century and the subject of a patent application by Endo for an analgesic and antitussive. References {{Opioidergics 4,5-Epoxym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Drug Metabolites
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, and language. Humans are highly social and tend to live in complex social structures composed of many cooperating and competing groups, from families and kinship networks to political states. Social interactions between humans have established a wide variety of values, social norms, and rituals, which bolster human society. Its intelligence and its desire to understand and influence the environment and to explain and manipulate phenomena have motivated humanity's development of science, philosophy, mythology, religion, and other fields of study. Although some scientists equate the term ''humans'' with all members of the genus ''Homo'', in common usage, it generally refers to ''Homo sapiens'', the only extant member. Anatomically mode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |