|
Malonyl-CoA Decarboxylase
Malonyl-CoA decarboxylase (), (which can also be called MCD and malonyl-CoA carboxyl-lyase) is found in bacteria and humans and has important roles in regulating fatty acid metabolism and food intake, and it is an attractive target for drug discovery. It is an enzyme associated with Malonyl-CoA decarboxylase deficiency. In humans, it is encoded by the MLYCD gene. Its main function is to catalyze the conversion of malonyl-CoA into acetyl-CoA and carbon dioxide. It is involved in fatty acid biosynthesis. To some degree, it reverses the action of Acetyl-CoA carboxylase. Structure MCD presents two isoforms which can be transcribed form one gene: a long isoform (54kDa), distributed in mitochondria, and a short isoform (49kDa) that can be found in peroxisomes and cytosol. The long isoform includes a sequence of signaling towards mitochondria in the N-terminus; whereas the short one only contains the typical sequence of peroxisomal signaling PTS1 in the C-terminus, also shared by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA Decarboxylase Deficiency
Malonyl-CoA decarboxylase deficiency (MCD) is an autosomal-recessive metabolic disorder caused by a genetic mutation that disrupts the activity of Malonyl-CoA decarboxylase. This enzyme breaks down Malonyl-CoA (a fatty acid precursor and a fatty acid oxidation blocker) into acetyl-CoA and carbon dioxide. Signs and symptoms The signs and symptoms of this disorder typically appear in early childhood. Almost all affected children have delayed development. Additional signs and symptoms can include weak muscle tone (hypotonia), seizures, diarrhea, vomiting, and low blood sugar (hypoglycemia). A heart condition called cardiomyopathy, which weakens and enlarges the heart muscle, is another common feature of malonyl-CoA decarboxylase deficiency. Some common symptoms in Malonyl-CoA decarboxylase deficiency, such as cardiomyopathy and metabolic acidosis, are triggered by the high concentrations of Malonyl-CoA in the cytoplasm. High levels of Malonyl-CoA will inhibit β-oxidation of fatty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcription (genetics)
Transcription is the process of copying a segment of DNA into RNA. The segments of DNA transcribed into RNA molecules that can encode proteins are said to produce messenger RNA (mRNA). Other segments of DNA are copied into RNA molecules called non-coding RNAs (ncRNAs). mRNA comprises only 1–3% of total RNA samples. Less than 2% of the human genome can be transcribed into mRNA ( Human genome#Coding vs. noncoding DNA), while at least 80% of mammalian genomic DNA can be actively transcribed (in one or more types of cells), with the majority of this 80% considered to be ncRNA. Both DNA and RNA are nucleic acids, which use base pairs of nucleotides as a complementary language. During transcription, a DNA sequence is read by an RNA polymerase, which produces a complementary, antiparallel RNA strand called a primary transcript. Transcription proceeds in the following general steps: # RNA polymerase, together with one or more general transcription factors, binds to promoter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SIRT4
Sirtuins are a family of signaling proteins involved in metabolic regulation. They are ancient in animal evolution and appear to possess a highly conserved structure throughout all kingdoms of life. Chemically, sirtuins are a class of proteins that possess either mono- ADP-ribosyltransferase or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity. The name Sir2 comes from the yeast gene 'silent mating-type information regulation 2', the gene responsible for cellular regulation in yeast. From ''in vitro'' studies, sirtuins are implicated in influencing cellular processes like aging, transcription, apoptosis, inflammation and stress resistance, as well as energy efficiency and alertness during low-calorie situations. As of 2018, there was no clinical evidence that sirtuins affect human aging. Yeast Sir2 and some, but not all, sirtuins are protein deacetylases. Unlike other known protein deacetylases, which simply hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deacetylation
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: * Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. * Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. * Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3CO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylation
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: *Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. * Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. * Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3CO)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called gly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
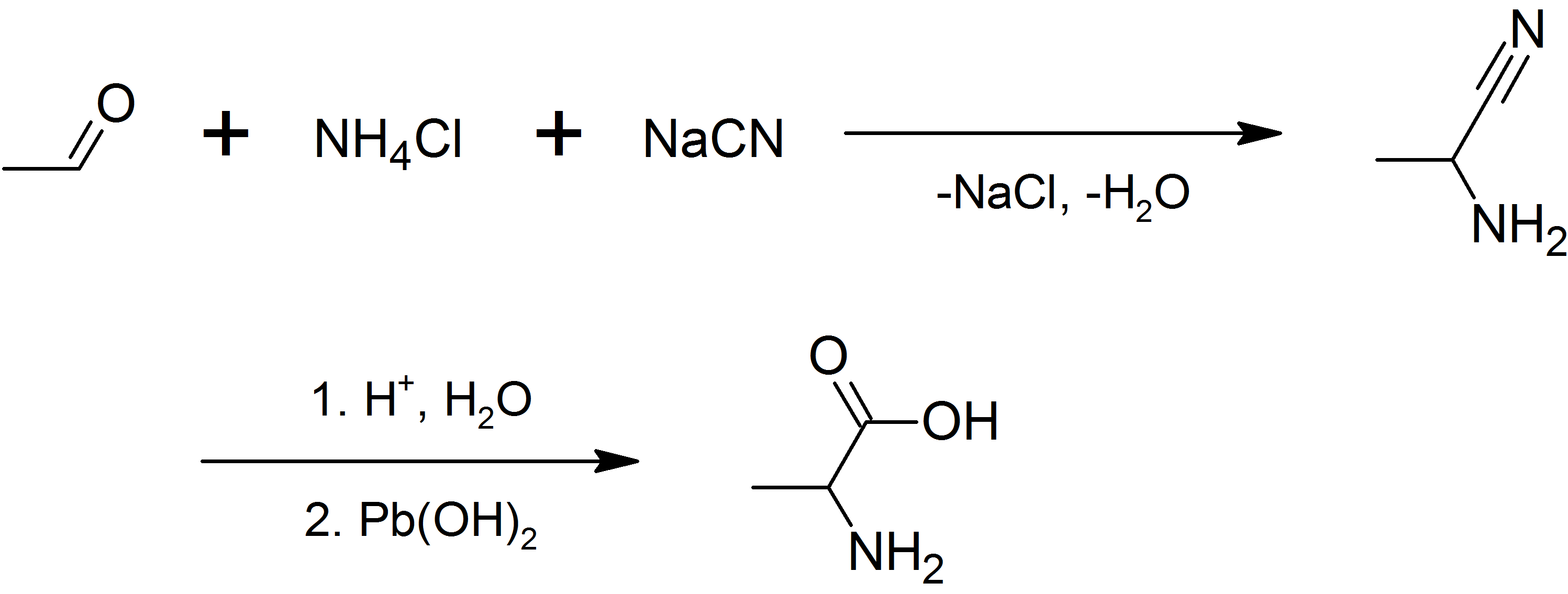
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16-30 amino acids long) present at the N-terminus (or occasionally nonclassically at the C-terminus or internally) of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles (the endoplasmic reticulum, Golgi or endosomes), secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, the majority of type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide. Function (translocation) Signal peptides function to prompt a cell to tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit. The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesised. Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen. Pepsinogen is activated when chief ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Precursor
A protein precursor, also called a pro-protein or pro-peptide, is an inactive protein (or peptide) that can be turned into an active form by post-translational modification, such as breaking off a piece of the molecule or adding on another molecule. The name of the precursor for a protein is often prefixed by ''pro-''. Examples include proinsulin and proopiomelanocortin, which are both prohormones. Protein precursors are often used by an organism when the subsequent protein is potentially harmful, but needs to be available on short notice and/or in large quantities. Enzyme precursors are called zymogens or proenzymes. Examples are enzymes of the digestive tract in humans. Some protein precursors are secreted from the cell. Many of these are synthesized with an N-terminal signal peptide that targets them for secretion. Like other proteins that contain a signal peptide, their name is prefixed by ''pre''. They are thus called pre-pro-proteins or pre-pro-peptides. The signal peptide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |