|
Malcolm Dixon
Malcolm Dixon (18 April 1899 – 7 December 1985) was a British biochemist. Education and early life Dixon was born in Cambridge, UK to Allick Page Dixon and Caroline Dewe Dixon (née Mathews). He received his PhD in 1925, for research supervised by Frederick Gowland Hopkins at the University of Cambridge. Research and career Dixon's research investigated the purification of enzymes and the enzyme kinetics of enzyme-catalyzed reactions. He studied the oxidation of glutathione and other thiols by molecular oxygen and measured the redox potential of the thiol-disulfide system, also establishing that the oxidation of glutathione was catalyzed by trace metals. He investigated xanthine oxidase, and thereby elucidated many aspects of the chemistry of dehydrogenases. He showed that the hydrogen peroxide formed in the reaction of xanthine oxidase with molecular oxygen inactivated the enzyme and that the inhibition could be relieved by the addition of catalase, thus helping to establish ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cambridge
Cambridge ( ) is a university city and the county town in Cambridgeshire, England. It is located on the River Cam approximately north of London. As of the 2021 United Kingdom census, the population of Cambridge was 145,700. Cambridge became an important trading centre during the Roman and Viking ages, and there is archaeological evidence of settlement in the area as early as the Bronze Age. The first town charters were granted in the 12th century, although modern city status was not officially conferred until 1951. The city is most famous as the home of the University of Cambridge, which was founded in 1209 and consistently ranks among the best universities in the world. The buildings of the university include King's College Chapel, Cavendish Laboratory, and the Cambridge University Library, one of the largest legal deposit libraries in the world. The city's skyline is dominated by several college buildings, along with the spire of the Our Lady and the English Martyrs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
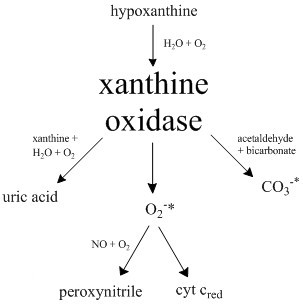
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUBMB
The International Union of Biochemistry and Molecular Biology (IUBMB) is an international non-governmental organisation concerned with biochemistry and molecular biology. Formed in 1955 as the International Union of Biochemistry (IUB), the union has presently 79 member countries and regions (as of 2020).IUBMB: the first half-century. /ref> The Union is devoted to promoting research and education in biochemistry and molecular biology throughout the world and gives particular attention to areas where the subject is still in its early development History The first Congress of Biochemistry ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mustard Gas
Mustard gas or sulfur mustard is a chemical compound belonging to a family of cytotoxic and blister agents known as mustard agents. The name ''mustard gas'' is technically incorrect: the substance, when dispersed, is often not actually a gas, but is instead in the form of a fine mist of liquid droplets.https://www.acs.org/content/dam/acsorg/education/resources/highschool/chemmatters/gc-mustard-gas-personal-safety-and-natl-security.pdf Mustard gases form blisters on exposed skin and in the lungs, often resulting in prolonged illness ending in death. The active ingredient in typical mustard gas is the organosulfur compound bis(2-chloroethyl) sulfide. In the wider sense, compounds with the structural element are known as ''sulfur mustards'' and ''nitrogen mustards'', respectively. Such compounds are potent alkylating agents, which can interfere with several biological processes. History as chemical weapons As a chemical weapon, mustard gas was first used in World War I, and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymator
Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray ( nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide and Mace (a branded mixture). While lachrymatory agents are commonly deployed for riot control by law enforcement and military personnel, its use in warfare is prohibited by various international treaties.E.g. the Geneva Protocol of 1925 prohibited the use of "asphyxiating gas, or any other kind of gas, liquids, substances or similar materials". During World War I, increasingly toxic and deadly lachrymatory agents were used. The short and long-te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C
The cytochrome complex, or cyt ''c'', is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins and plays a major role in cell apoptosis. Cytochrome c is highly water-soluble, unlike other cytochromes, and is an essential component of the respiratory electron transport chain, where it carries one electron. It is capable of undergoing oxidation and reduction as its iron atom converts between the ferrous and ferric forms, but does not bind oxygen. It transfers electrons between Complexes III (Coenzyme Q – Cyt c reductase) and IV (Cyt c oxidase). In humans, cytochrome c is encoded by the ''CYCS'' gene. Species distribution Cytochrome c is a highly conserved protein across the spectrum of eukaryotic species, found in plants, animals, fungi, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robin Hill (biochemist)
Robert Hill FRS (2 April 1899 – 15 March 1991), known as Robin Hill, was a British plant biochemist who, in 1939, demonstrated the ' Hill reaction' of photosynthesis, proving that oxygen is evolved during the light requiring steps of photosynthesis. He also made significant contributions to the development of the Z-scheme of oxygenic photosynthesis. Education and early life Hill was born in New Milverton, a suburb of Leamington Spa, Warwickshire. He was educated at Bedales School, where he became interested in biology and astronomy (he published a paper on sunspots in 1917), and Emmanuel College, Cambridge, where he read Natural Sciences. During the First World War he served in the Anti-gas Department of the Royal Engineers. Career In 1922, he joined the Department of Biochemistry at Cambridge, where he was directed to research hemoglobin. He published a number of papers on hemoglobin, and in 1926 he began to work with David Keilin on the haem containing protein cytochrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
David Keilin
David Keilin FRS (21 March 1887 – 27 February 1963) was a Jewish scientist focusing mainly on entomology. Background and education He was born in Moscow in 1887 and his family returned to Warsaw early in his youth. He did not attend school until age ten due to ill health and asthma. Only seven years later, in 1904, he enrolled in the University of Liège. He later studied at Magdalene College, Cambridge, and became a British citizen. Career Keilin became research assistant to George Nuttall, first Quick Professor of Biology at the University of Cambridge, in 1915, and spent the rest of his career there, succeeding Nuttall as Quick Professor and director of the Molteno Institute in 1931. He retired in 1952. He made extensive contributions to entomology and parasitology during his career. He published thirty-nine papers between 1914 and 1923 on the reproduction of lice, the life-cycle of the horse bot-fly, the respiratory adaptations in fly larvae, and other subjects. He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction rat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme
A cofactor is a non- protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidase
In biochemistry, an oxidase is an enzyme that catalyzes oxidation-reduction reactions, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydrogen peroxide (H2O2). Some oxidation reactions, such as those involving monoamine oxidase or xanthine oxidase, typically do not involve free molecular oxygen. The oxidases are a subclass of the oxidoreductases. Examples An important example is cytochrome c oxidase, the key enzyme that allows the body to employ oxygen in the generation of energy and the final component of the electron transfer chain. Other examples are: * Glucose oxidase * Monoamine oxidase * Cytochrome P450 oxidase * NADPH oxidase * Xanthine oxidase * L-gulonolactone oxidase * Laccase * Lysyl oxidase * Polyphenol oxidase * Sulfhydryl oxidase. This enzyme oxidises thiol groups. Oxidase test In microbiology, the oxidase test is used as a phenotypic characteristic for the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)


