|
Magnesium Carbonate
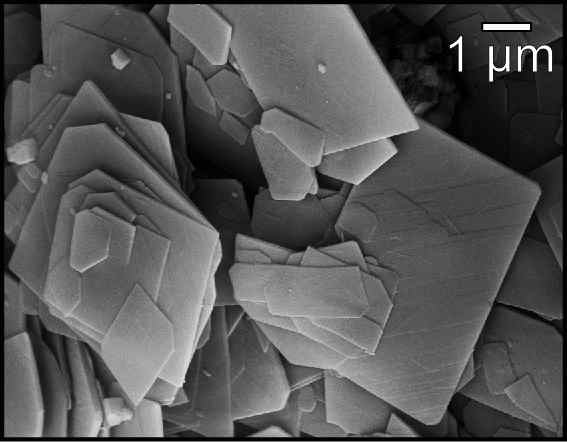
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals. Forms The most common magnesium carbonate forms are the anhydrous salt called magnesite (), and the di, tri, and pentahydrates known as barringtonite (), nesquehonite (), and lansfordite (), respectively. Some basic forms such as artinite (), hydromagnesite (), and dypingite () also occur as minerals. All of those minerals are colouress or white. Magnesite consists of colourless or white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react with acids. Magnesite crystallizes in the calcite structure wherein is surrounded by six oxygen atoms. The dihydrate has a triclinic structure, while the trihydrate has a monoclinic structure. References to "light" and "heavy" magnesium carbonates actually refer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesite
Magnesite is a mineral with the chemical formula (magnesium carbonate). Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts. Occurrence Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites are often cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide in groundwaters. Isotopic structure: clumped isotope The recent advancement in the field of stable isotope geochemistry is the study of isotopic structure of minerals and molecules. This requires study of molecules with high resolutions looking at bonding scenario (how heavy isotopes are bonded to each other)- leading to kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minerals
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dypingite
Dypingite is a hydrated magnesium carbonate mineral with the formula: Mg5(CO3)4(OH)2·5H2O. Its type locality is the Dypingdal serpentine-magnesite deposit, Snarum, Norway Norway, officially the Kingdom of Norway, is a Nordic country in Northern Europe, the mainland territory of which comprises the western and northernmost portion of the Scandinavian Peninsula. The remote Arctic island of Jan Mayen and t .... References Magnesium minerals Carbonate minerals Monoclinic minerals {{carbonate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydromagnesite
Hydromagnesite is a hydrated magnesium carbonate mineral with the formula Mg5(CO3)4(OH)2·4H2O. It generally occurs associated with the weathering products of magnesium containing minerals such as serpentine or brucite. It occurs as incrustations and vein or fracture fillings in ultramafic rocks and serpentinites, and occurs in hydrothermally altered dolomite and marble. Hydromagnesite commonly appears in caves as speleothems and "moonmilk", deposited from water that has seeped through magnesium rich rocks. It is the most common cave carbonate after calcite and aragonite. The mineral thermally decomposes, over a temperature range of approximately 220 °C to 550 °C, releasing water and carbon dioxide leaving a magnesium oxide residue. Hydromagnesite was first described in 1836 for an occurrence in Hoboken, New Jersey. Stromatolites in an alkaline ( pH greater than 9) freshwater lake ( Salda Gölü) in southern Turkey are made of hydromagnesite precipitated by diatoms a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artinite
Artinite is a hydrated magnesium carbonate mineral with formula: Mg2(CO3)(OH)2·3H2O. It forms white silky monoclinic prismatic crystals that are often in radial arrays or encrustations. It has a Mohs hardness of 2.5 and a specific gravity of 2. It occurs in low-temperature hydrothermal veins and in serpentinized ultramafic rocks. Associated minerals include brucite, hydromagnesite, pyroaurite, chrysotile, aragonite, calcite, dolomite and magnesite. It was first reported in 1902 in Lombardy, Italy. It was named for Italian mineralogist, Ettore Artini Ettore is a given name, the Italian version of Hector. People *Ettore Arrigoni degli Oddi (1867–1942), Italian naturalist *Ettore Bassi (born 1970), Italian actor and television presenter *Ettore Bastianini (1922–1967), Italian opera singer *Et ... (1866–1928). References Magnesium minerals Carbonate minerals Monoclinic minerals Minerals in space group 12 {{carbonate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesite
Magnesite is a mineral with the chemical formula (magnesium carbonate). Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts. Occurrence Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites are often cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide in groundwaters. Isotopic structure: clumped isotope The recent advancement in the field of stable isotope geochemistry is the study of isotopic structure of minerals and molecules. This requires study of molecules with high resolutions looking at bonding scenario (how heavy isotopes are bonded to each other)- leading to kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when performi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica .... A base was therefore a metal hydroxide such as Sodium hydroxide, NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |