|
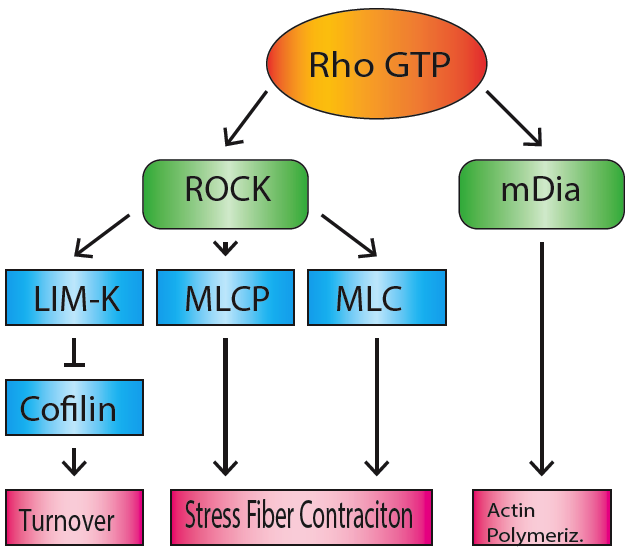
MDia1
mDia1 (also known as Dia1, Drf1 for Diaphanous-related formin-1, Diaph1, KIAA4062, p140mDia, mKIAA4062, or D18Wsu154e) is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends (fast-growing ends) of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named ''Diap1''. mDia1 is highly homologous to Drosophila diaphanous, regulating the cytokinetic ring during cytokinesis. Homologues in other species are known as well, like the human DIAP1, budding yeast Bni1 or fission yeast Cdc12p. The gene has been knocked-out in mice. Structure The product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mdia1 Domains
mDia1 (also known as Dia1, Drf1 for Diaphanous-related formin-1, Diaph1, KIAA4062, p140mDia, mKIAA4062, or D18Wsu154e) is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends (fast-growing ends) of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named ''Diap1''. mDia1 is highly homologous to Drosophila diaphanous, regulating the cytokinetic ring during cytokinesis. Homologues in other species are known as well, like the human DIAP1, budding yeast Bni1 or fission yeast Cdc12p. The gene has been knocked-out in mice. Structure The product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formins
Formins (formin homology proteins) are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end (barbed end) of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus. Diversity Formins have been found in all eukaryotes studied. In humans, 15 different formin proteins are present that have been classified in 7 subgroups. By contrast, yeasts contain only 2-3 formins. Structure and interactions Formins are characterized by the presence of three formin homology (FH) domains (FH1, FH2 and FH3), although members of the formin family do not ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formins
Formins (formin homology proteins) are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end (barbed end) of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus. Diversity Formins have been found in all eukaryotes studied. In humans, 15 different formin proteins are present that have been classified in 7 subgroups. By contrast, yeasts contain only 2-3 formins. Structure and interactions Formins are characterized by the presence of three formin homology (FH) domains (FH1, FH2 and FH3), although members of the formin family do not ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formin
Formins (formin homology proteins) are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end (barbed end) of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus. Diversity Formins have been found in all eukaryotes studied. In humans, 15 different formin proteins are present that have been classified in 7 subgroups. By contrast, yeasts contain only 2-3 formins. Structure and interactions Formins are characterized by the presence of three formin homology (FH) domains (FH1, FH2 and FH3), although members of the formin family do not necessa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress Fiber
Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis. Structure Stress fibers are primarily composed of actin and myosin. Actin is a ~43kDa globular protein, and can polymerize to form long filamentous structures. These filaments are made of two strands of actin monomers (or protofilaments) wrapping around each other, to create a single actin filament. Because actin monomers are not symmetrical molecules, their filaments have polarity based upon the structure of the actin monomer, which will allow one end of the actin filament to polymerize faster than the other. The end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress Fiber
Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis. Structure Stress fibers are primarily composed of actin and myosin. Actin is a ~43kDa globular protein, and can polymerize to form long filamentous structures. These filaments are made of two strands of actin monomers (or protofilaments) wrapping around each other, to create a single actin filament. Because actin monomers are not symmetrical molecules, their filaments have polarity based upon the structure of the actin monomer, which will allow one end of the actin filament to polymerize faster than the other. The end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
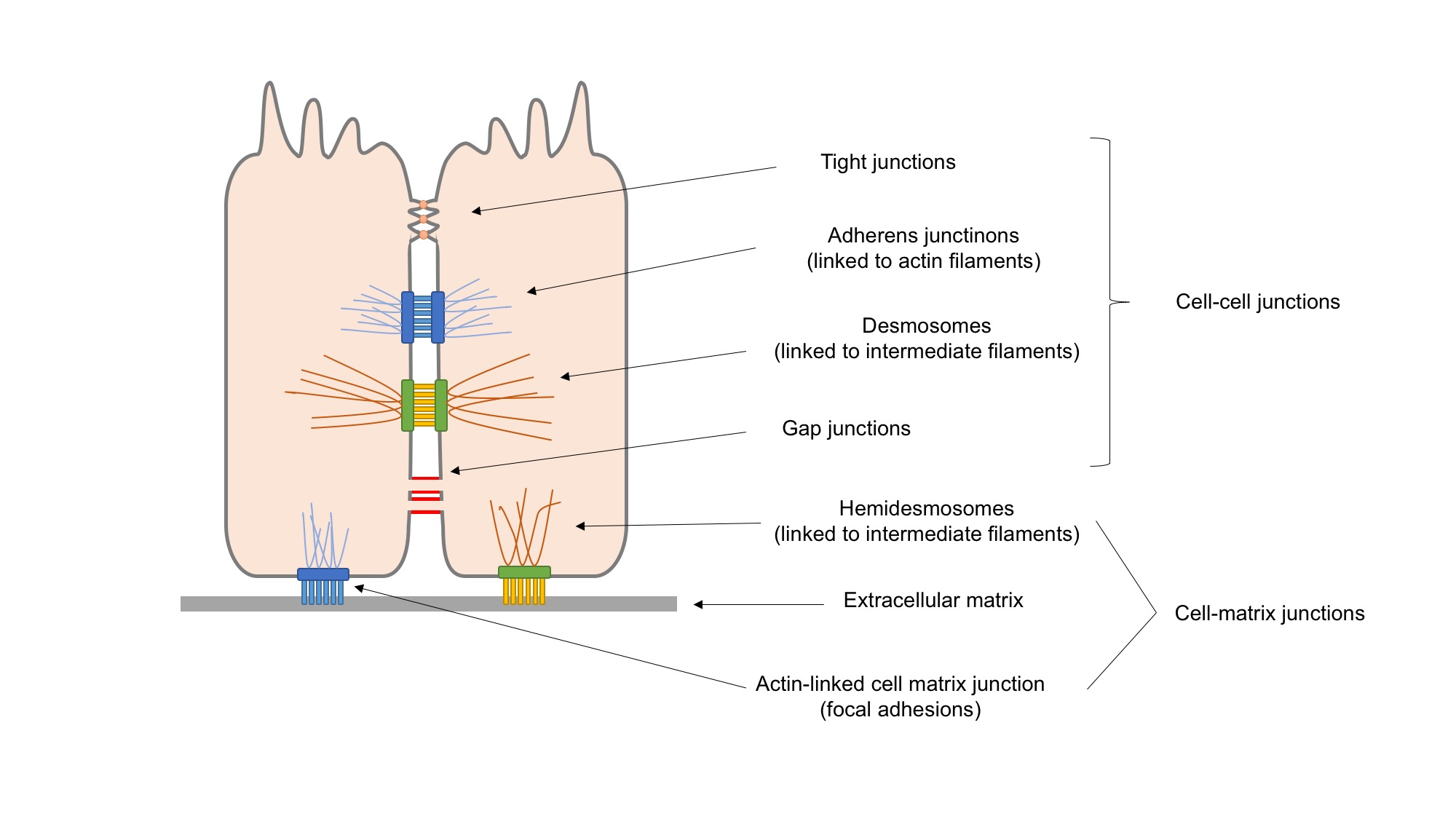
Focal Adhesion
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IQGAP1
Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the ''IQGAP1'' gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle. History IQGAP1 was discovered in 1994. Its name stems from the fact that its RasGAP-related domain (GRD) has sequence homology to the Sar1 GTPase. It was hypothesized that IQGAP1 would act as a GTPase activating protein (GAP) protein, promoting the switch of ras GTPases from the active GTP to GDP-bound forms. However, despite the homology of IQGAP’s GAP domain to sar1 and the fact that IQGAP1 binds Rho GTPases Rac1 and Cdc42, IQGAP does not actually have GAP function. Instead, it binds the active (GTP-bound) forms of RAC1 and CDC42 with higher affinity than GDP-bound forms, and stabilizes the active form in vivo. IQGAP1 is now ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Adhesion
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endocytosis
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis (cell drinking) and phagocytosis (cell eating). It is a form of active transport. History The term was proposed by De Duve in 1963. Phagocytosis was discovered by Élie Metchnikoff in 1882. Pathways Endocytosis pathways can be subdivided into four categories: namely, receptor-mediated endocytosis (also known as clathrin-mediated endocytosis), caveolae, pinocytosis, and phagocytosis. * Clathrin-mediated endocytosis is mediated by the production of small (approx. 100 nm in diameter) vesicles that have a morphologically characteristic coat made up of the cytosolic protein clathrin. Clathrin-coated vesicles (CCVs) are found in virtually all cells and form domains of the plasma membrane ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho Family Of GTPases
The Rho family of GTPases is a family of small (~21 kDa) signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions. History Identification of the Rho family of GTPases began in the mid-1980s. The first identified Rho member was RhoA, isolated serendipitously in 1985 from a low stringency cDNA screening. Rac1 and Rac2 were identified next, in 1989 followed by Cdc42 in 1990. Eight additional mammalian Rho members were identified from biological screenings until the late 1990s, a turning point in biology where availability of complete genome seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |