|
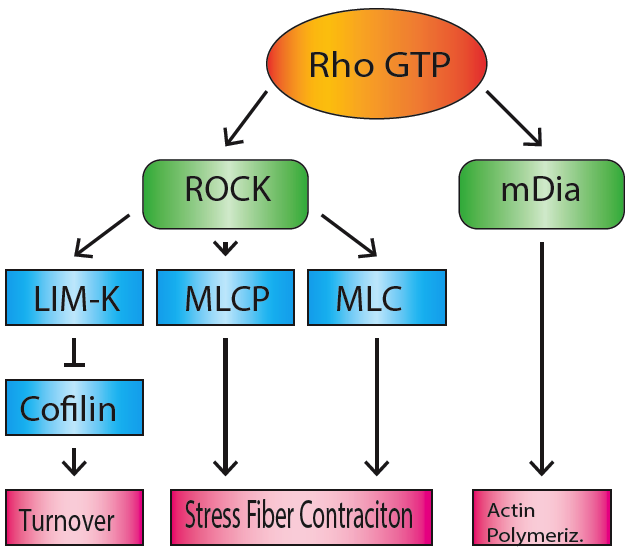
Stress Fiber
Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis. Structure Stress fibers are primarily composed of actin and myosin. Actin is a ~43kDa globular protein, and can polymerize to form long filamentous structures. These filaments are made of two strands of actin monomers (or protofilaments) wrapping around each other, to create a single actin filament. Because actin monomers are not symmetrical molecules, their filaments have polarity based upon the structure of the actin monomer, which will allow one end of the actin filament to polymerize faster than the other. The end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement, cell sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadherin
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that is important in the formation of adherens junctions to allow cells to adhere to each other . Cadherins are a class of type-1 transmembrane proteins, and they are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred to as the cadherin adhesome. The cadherin family is essential in maintaining the cell-cell contact and regulating cytoskeletal complexes. The cadherin superfamily includes cadherins, protocadherins, desmogleins, desmocollins, and more. In structure, they share ''cadherin repeats'', which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecules, each designated with a prefix (in general, noting the types of tissue with which it is associated). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
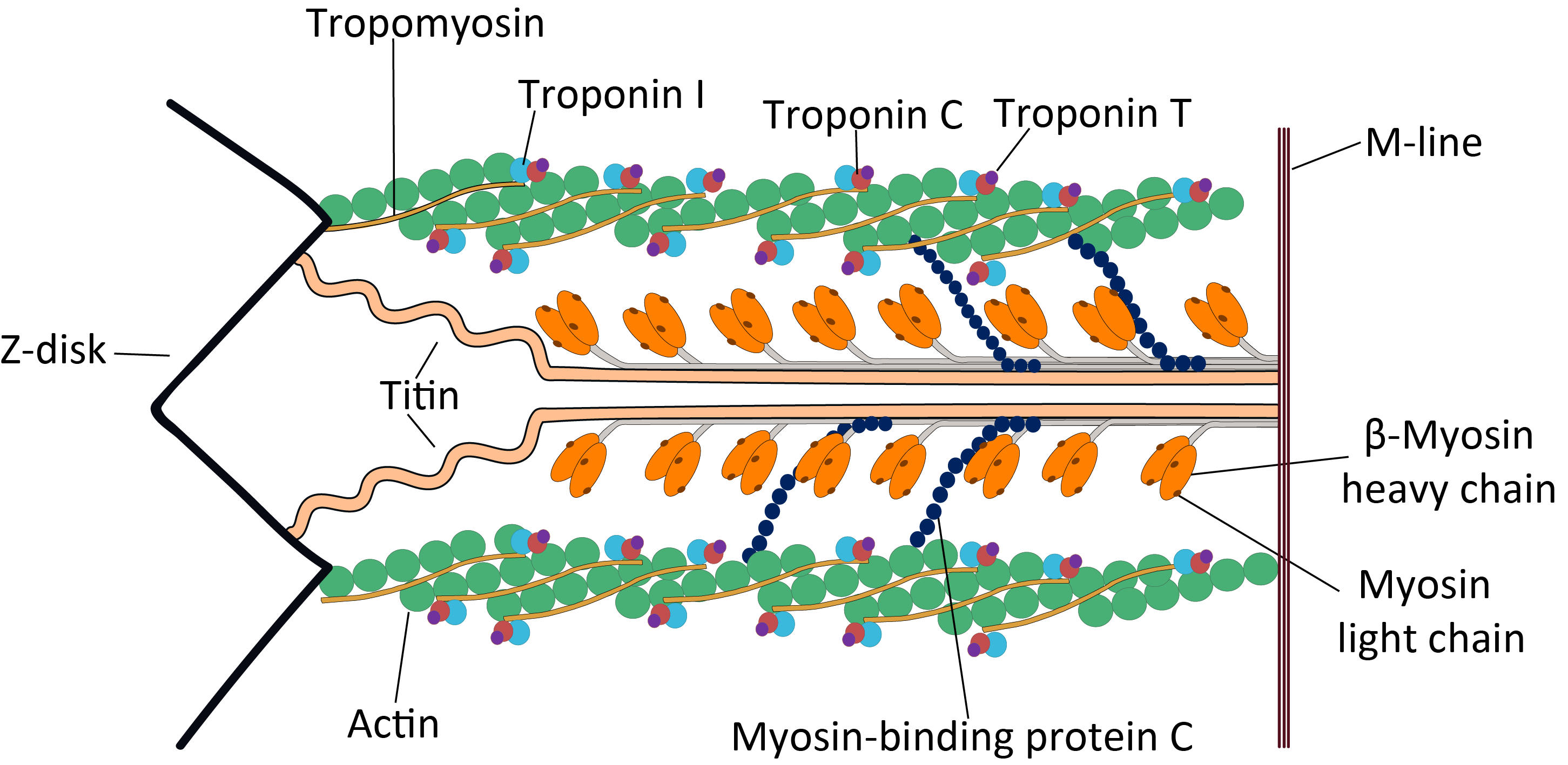
Sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. Myos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LINC Complex
The LINC complex (Linker of Nucleoskeleton and Cytoskeleton) is a protein complex associated with both inner and outer membranes of the nucleus. It is composed of SUN-domain proteins and KASH-domain proteins. The SUN-domain proteins are associated with both nuclear lamins and chromatin and cross the inner nuclear membrane. They interact with the KASH domain KASH domains are conserved C-terminal protein regions less than ~30 amino acids. KASH is an acronym for Klarsicht, ANC-1, Syne Homology. KASH domains always follow a transmembrane domain. Most proteins containing KASH domains are thought to be in ... proteins in the perinuclear (lumen) space between the two membranes. The KASH domain proteins cross the outer nuclear membrane and interact with actin filaments, microtubule filaments (through dynein and kinesin motors), intermediate filaments (through spectrin), centrosomes and cytoplasmic organelles. The number of SUN-domain and KASH-domain proteins increased in evolution. Func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
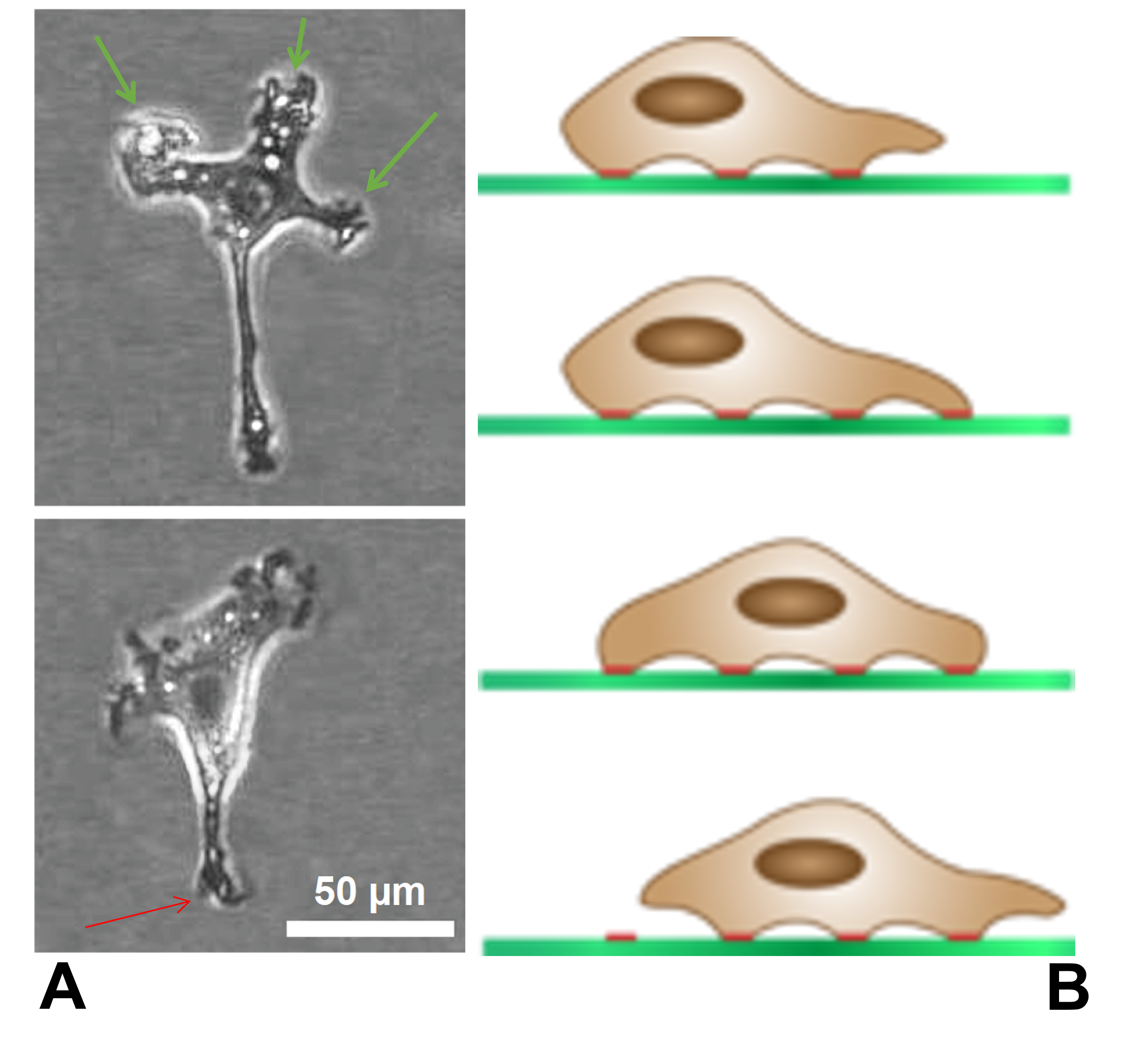
Filopodia
Filopodia (singular filopodium) are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. Within the lamellipodium, actin ribs are known as ''microspikes'', and when they extend beyond the lamellipodia, they're known as filopodia. They contain microfilaments (also called actin filaments) cross-linked into bundles by actin-bundling proteins, such as fascin and fimbrin. Filopodia form focal adhesions with the substratum, linking them to the cell surface. Many types of migrating cells display filopodia, which are thought to be involved in both sensation of chemotropic cues, and resulting changes in directed locomotion. Activation of the Rho family of GTPases, particularly cdc42 and their downstream intermediates, results in the polymerization of actin fibers by Ena/Vasp homology proteins. Growth factors bind to receptor tyrosine kinases resulting in the polymerization of actin filaments, which, when cross-linked, make up the sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamellipodium
The lamellipodium (plural lamellipodia) (from Latin ''lamella'', related to ', "thin sheet", and the Greek radical ''pod-'', "foot") is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell. Description Lamellipodia are found primarily in all mobile cells, such as the keratinocytes of fish and frogs, which are involved in the quick repair of wounds. The lamellipodia of these keratinocytes allow them to move at speeds of 10–20 μm / min over epithelial surfaces. When separated from the main part of a cell, a lamellipodium can still cra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotaxis
Chemotaxis (from '' chemo-'' + ''taxis'') is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food (e.g., glucose) by swimming toward the highest concentration of food molecules, or to flee from poisons (e.g., phenol). In multicellular organisms, chemotaxis is critical to early development (e.g., movement of sperm towards the egg during fertilization) and development (e.g., migration of neurons or lymphocytes) as well as in normal function and health (e.g., migration of leukocytes during injury or infection). In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Durotaxis
Durotaxis is a form of cell migration in which cells are guided by rigidity gradients, which arise from differential structural properties of the extracellular matrix (ECM). Most normal cells migrate up rigidity gradients (in the direction of greater stiffness). History of durotaxis research The process of durotaxis requires a cell to actively sense the environment, process the mechanical stimulus, and execute a response. Originally, this was believed to be an emergent metazoan property, as the phenomenon requires a complex sensory loop that is dependent on the communication of many different cells. However, as the wealth of relevant scientific literature grew in the late 1980s and throughout the 1990s, it became apparent that single cells possess the ability to do the same. The first observations of durotaxis in isolated cells were that mechanical stimuli could cause the initiation and elongation of axons in the sensory and brain neurons of chicks and induce motility in previousl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress Fibers - Three Types
Stress may refer to: Science and medicine * Stress (biology), an organism's response to a stressor such as an environmental condition * Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase or sentence * Stress (mechanics), the internal forces that neighboring particles of a continuous material exert on each other * Occupational stress, stress related to one's job * Psychological stress, a feeling of strain and pressure * Surgical stress, systemic response to surgical injury Arts, entertainment, and media Music Groups and musicians * Stress (Brazilian band), a Brazilian heavy metal band * Stress (British band), a British rock band * Stress (pop rock band), an early 1980s melodic rock band from San Diego * Stress (musician) (born 1977), hip hop singer from Switzerland * Stress (record producer) (born 1979), artistic name of Can Canatan, Swedish musician and record producer Albums * ''Stress'' (Anonymus album), 1997 * ''St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Adhesion
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tight Junction
Tight junctions, also known as occluding junctions or ''zonulae occludentes'' (singular, ''zonula occludens''), are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals between the epithelial cells. They also play a critical role maintaining the structure and permeability of endothelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. The corresponding junctions that occur in invertebrates are septate junctions. Structure Tight junctions are composed of a branching network of sealing strands, each strand acting independently from the others. Therefore, the efficiency of the junction in preventing ion passage increases exponentially with the number of strands. Each strand is formed from a row of transmembrane proteins embedded in both plasma membranes, with extracellular domains joining one another directly. There are at least 40 different protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
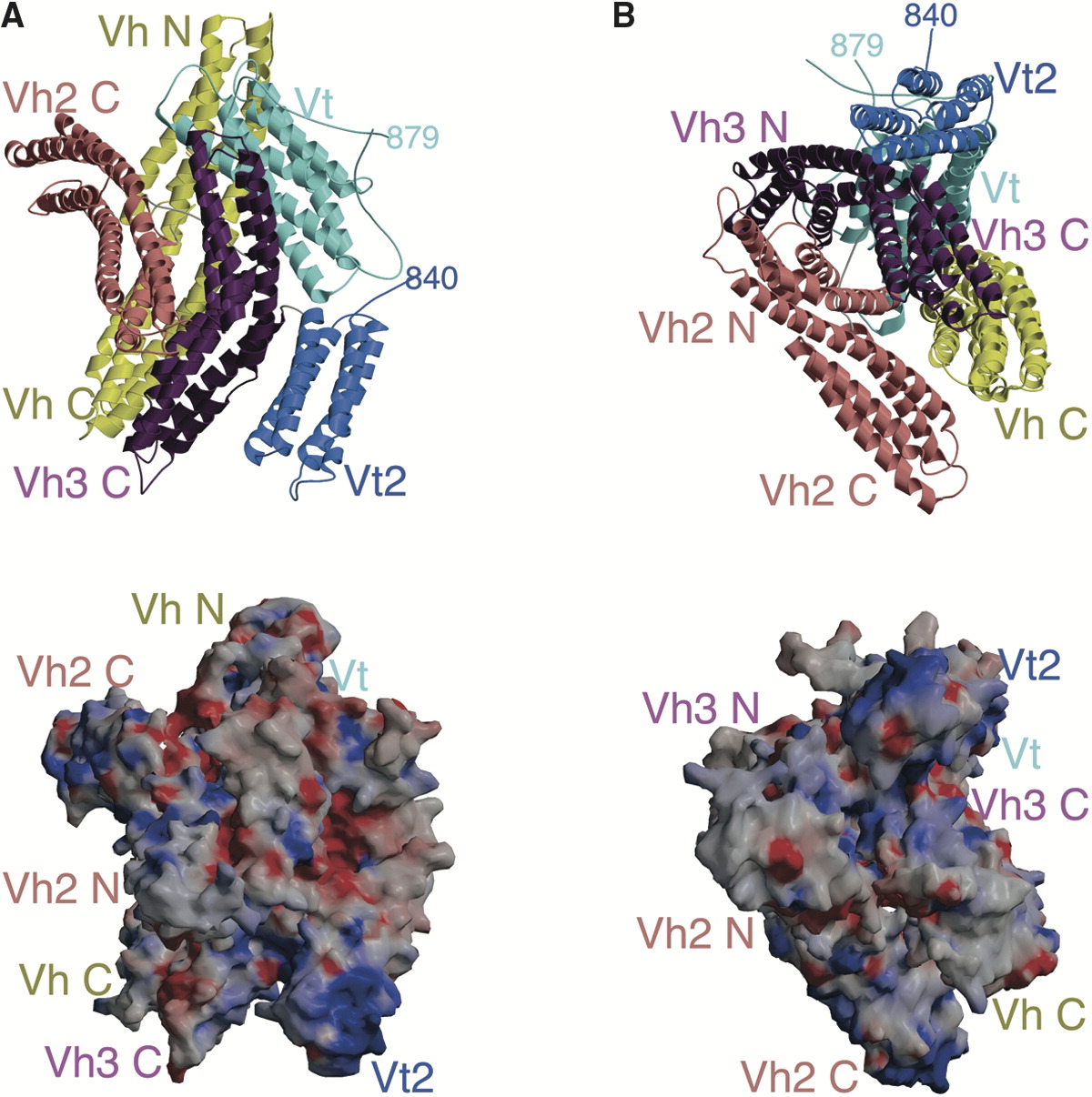
Vinculin
In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane. Discovered independently by Benny Geiger and Keith Burridge, its sequence is 20%–30% similar to α-catenin, which serves a similar function. Binding alternately to talin or α-actinin, vinculin's shape and, as a consequence, its binding properties are changed. The vinculin gene occurs as a single copy and what appears to be no close relative to take over functions in its absence. Its splice variant metavinculin (see below) also needs vinculin to heterodimerize and work in a dependent fashion. Structure Vinculin is a 117-kDa cytoskeletal protein with 1066 amino acids. The protein contains an acidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |