|
Myers Allene Synthesis
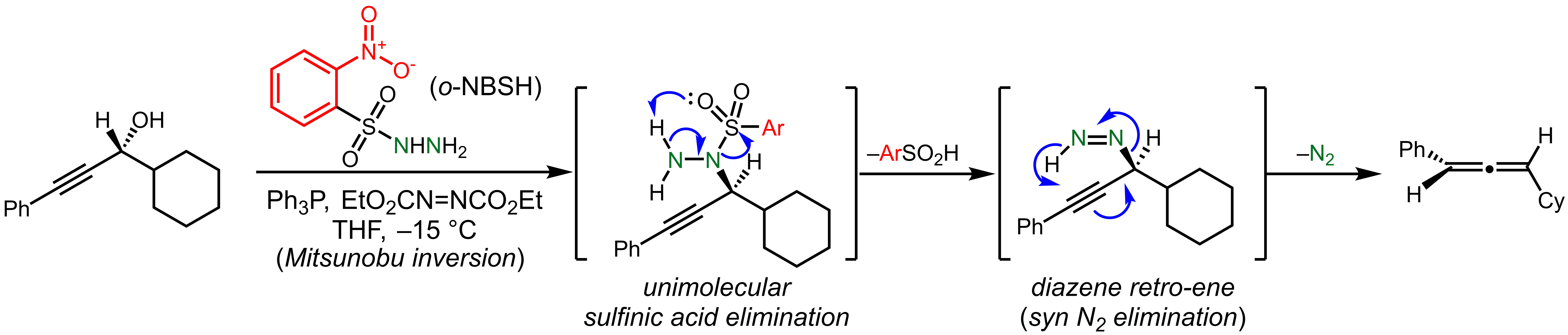
In organic chemistry, the Myers allene synthesis is a chemical reaction that converts a propargyl alcohol into an allene by way of an arenesulfonylhydrazine as a key intermediate. This name reaction is one of two discovered by Andrew Myers that are named after him; both this reaction and the Myers deoxygenation reaction involve the same type of intermediate. The reaction is a three-step process in which the alcohol first undergoes a Mitsunobu reaction with an arenesulfonylhydrazine in the presence of triphenylphosphine and diethyl azodicarboxylate. Unlike hydrazone-synthesis reactions, this reaction occurs on the same nitrogen of the hydrazine that has the arenesulfonyl substituent. Upon warming, this product undergoes an elimination of arylsulfinic acid to give an unstable diazene as a reactive intermediate. The diazene extrudes N2 to give isolated allene product. The authors describe this last step as a ,3sigmatropic reaction in the original report but call it a retro-ene react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfinic Acid
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal. Structure and properties Sulfinic acids RSO2H are about 1000x more acidic than the corresponding carboxylic acid RCO2H. Sulfur is pyramidal, consequently sulfinic acids are chiral. Preparation They are often prepared in situ by acidification of the corresponding sulfinate salts, which are typically more robust than the acid. These salts are generated by reduction of sulfonyl chlorides. An alternative route is the reaction of Grignard reagents with sulfur dioxide. Transition metal sulfinates are also generated by insertion of sulfur dioxide into metal alkyls, a reaction that may proceed via a metal sulfur dioxide complex. Unsubstituted sulfinic acid, when R is the hydrogen atom, is a higher energy isomer of sulfoxylic acid, both of which are unstable. Examples An example of a simple, well-studied sulfinic acid is phenylsulfinic acid. A commercially importa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis-trans'' notation does not always correspond to ''E''–''Z'' isomerism, which is an '' absolute'' stereochemical description. In general, ''cis''–''trans'' stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
One-pot
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds can save time and resources while increasing chemical yield. An example of a one-pot synthesis is the total synthesis of tropinone or the Gassman indole synthesis. Sequential one-pot syntheses can be used to generate even complex targets with multiple stereocentres, such as oseltamivir, which may significantly shorten the number of steps required overall and have important commercial implications. A sequential one-pot synthesis with reagents added to a reactor one at a time and without work-up is also called a telescoping synthesis. In one such procedure the reaction of 3-N-tosylaminophenol I with acrolein II affords a hydroxyl substituted quinoline III through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ''H'' = −117 kJ/mol). World capacity for nitrobenzene in 1985 was about 1,700,000 tonnes. The nitration process involves formation of the nitronium ion (NO2+), followed by an electrophilic aromatic substitution reaction of it with benzene. The nitronium ion is generated by the reaction of nitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ortho Substitution
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * In ''ortho''-substitution, two substituents occupy positions next to each other, which may be numbered 1 and 2. In the diagram, these positions are marked R and ''ortho''. * In ''meta''-substitution the substituents occupy positions 1 and 3 (corresponding to R and ''meta'' in the diagram). * In ''para''-substitution, the substituents occupy the opposite ends (positions 1 and 4, corresponding to R and ''para'' in the diagram). The toluidines serve as an example for these three types of substitution. Synthesis Electron donating groups, for example amino, hydroxyl, alkyl, and phenyl groups tend to be ''ortho''/''para''-directors, and electron withdrawing groups such as nitro, nitrile, and ketone groups, tend to be ''meta''-directors. Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter how t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereospecific
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation," Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. J. Am. Chem. Soc., 1959, 81, 108-116.Eliel, E., "Stereochemistry of Carbon Compound", McGraw-Hill, 1962 pp 434-436 In contrast, stereoselectivity is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as steric access, that are independent of the mechanism. A stereospecific mechanism ''specifies'' the stereochemical outcome of a given reactant, whereas a stereoselective reaction ''selects'' products from those made available by the same, non-specific mechanism acting on a gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
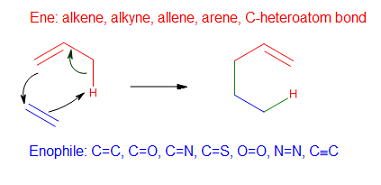
Ene Reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigmatropic Reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the long-established sigma designation from single carbon–carbon bonds and the Greek word ''tropos'', meaning turn. In this type of rearrangement reaction, a substituent moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the ,3 Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis. Overview of sigmatropic shifts Woodward–Hoffman sigmatropic shift nomenclatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |