Myers allene synthesis on:
[Wikipedia]
[Google]
[Amazon]
In 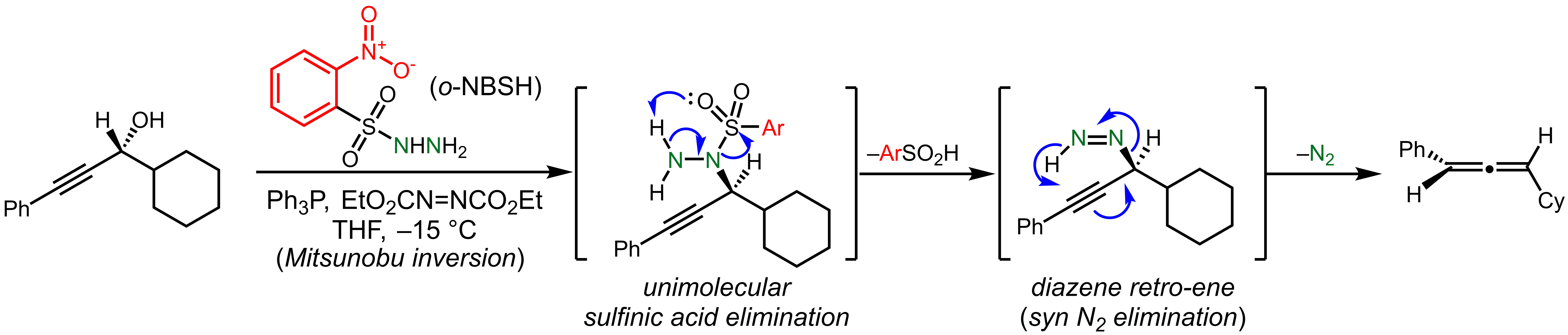 The use of '' ortho''-
The use of '' ortho''-
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, the Myers allene synthesis is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
that converts a propargyl alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the chemical formula, formula C3H4O. It is the simplest stable Alcohol (chemistry), alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid t ...
into an allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as diene#Classes, cumulated dienes. The parent compound of this class is propa ...
by way of an arenesulfonyl
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups c ...
hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
as a key intermediate. This name reaction A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include ...
is one of two discovered by Andrew Myers that are named after him; both this reaction and the Myers deoxygenation In organic chemistry, the Myers deoxygenation reaction is an organic redox reaction that reduces an alcohol into an alkyl position by way of an arenesulfonylhydrazine as a key intermediate. This name reaction is one of four discovered by Andrew My ...
reaction involve the same type of intermediate.
The reaction is a three-step process in which the alcohol first undergoes a Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylat ...
with an arenesulfonylhydrazine in the presence of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ...
and diethyl azodicarboxylate
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked ...
. Unlike hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
-synthesis reactions, this reaction occurs on the same nitrogen of the hydrazine that has the arenesulfonyl substituent. Upon warming, this product undergoes an elimination of arylsulfinic acid
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
Structure and properties
Sulfinic acids RSO2H are about 1000x more acidic than the corresponding carboxylic acid RCO2H. Su ...
to give an unstable diazene
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is ...
as a reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
. The diazene extrudes N2 to give isolated allene product. The authors describe this last step as a ,3sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one sigma bond, σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compound wor ...
in the original report but call it a retro-ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
in another publication. (''Note:'' The IUPAC defines a sigmatropic rearrangement as a pericyclic reaction involving both breaking and formation of a new σ bond in which the total number of π and σ bonds do not change, whereas a retro-ene reaction involves the fragmentation of a molecule to a fragment with a double bond with allylic hydrogen (the 'ene') and a multiple-bonded species (the 'enophile') via a cyclic transition state. In this case, the reaction occurs with the net gain of a π bond and loss of a σ bond, so strictly speaking, only the description of the reaction as a retro-ene reaction is apt.)
Both the first step (Mitsunobu reaction) and third step (sigmatropic reaction) are stereospecific
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Con ...
, so the chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
of the propargyl alcohol controls the chirality of the resulting allene.
 The use of '' ortho''-
The use of '' ortho''-nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
sulfonylhydrazine gives reactants and intermediates with appropriate relative stability to enable the whole process to be performed as a one-pot
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separ ...
reaction, though the order in which the reagents are mixed is important. Mechanistic studies suggest that the diazene is formed as mixture of ''cis'' and ''trans'' isomers that easily interconvert, and that the ''cis'' is what reacts most readily to form the allene.
References
{{organic-chem-stub Rearrangement reactions Name reactions