|
Mosesite
Mosesite is a very rare mineral found in few locations. It is a mercury mineral found as an accessory in deposits of mercury, often in conjunction with limestone. It is known to be found in the U.S. states of Texas and Nevada, and the Mexican states of Guerrero and Querétaro. It was named after Professor Alfred J. Moses (1859–1920) for his contributions to the field of mineralogy in discovering several minerals found alongside mosesite. The mineral itself is various shades of yellow and a high occurrence of spinel twinning. It becomes isotropic when heated to . Composition Mosesite contains 16 Hg, 3 Cl, SO4, CO3, MoO4, 16 H, and 8 N with a volume of 8.4777x10−1 nm3 and calculated density of 7.53 g/cm3. Its chemical formula is . Geologic occurrence Discovered in a mercury mine in Terlingua, Texas, mosesite has also been seen in Nevada and Mexico. Mosesite is a secondary mineral formed at low temperature in hydrothermal mercury deposits. The mercury ore at the mine in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide Minerals
Halide minerals are those minerals with a dominant halide anion (, , and ). Complex halide minerals may also have polyatomic anions. Examples include the following: *Atacamite * Avogadrite (K,Cs)BF *Bararite (β) *Bischofite * Brüggenite *Calomel *Carnallite *Carnallite * Cerargyrite/Horn silver AgCl * Chlorargyrite AgCl, bromargyrite AgBr, and iodargyrite AgI *Cryolite *Cryptohalite (a) Vanadates), 09 Silicates: * ''neso-'': insular (from Greek , "island") * ''soro-'': grouped (from Greek , "heap, pile, mound") * ''cyclo-'': ringed (from Greek , "circle") * ''ino-'': chained (from Greek , "fibre", rom Ancient Greek * ''phyllo-'': sheeted (from Greek , "leaf") * ''tecto-'': of three-dimensional framework (from Greek , "of building") ;Nickel–Strunz code scheme ''NN.XY.##x'': * ''NN'': Nickel–Strunz mineral class number * ''X'': Nickel–Strunz mineral division letter * ''Y'': Nickel–Strunz mineral family letter * ''##x'': Nickel–Strunz mineral/group n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleochroism
Pleochroism (from Greek πλέων, ''pléōn'', "more" and χρῶμα, ''khrôma'', "color") is an optical phenomenon in which a substance has different colors when observed at different angles, especially with polarized light. Background Anisotropic crystals will have optical properties that vary with the direction of light. The direction of the electric field determines the polarization of light, and crystals will respond in different ways if this angle is changed. These kinds of crystals have one or two optical axes. If absorption of light varies with the angle relative to the optical axis in a crystal then pleochroism results. Anisotropic crystals have double refraction of light where light of different polarizations is bent different amounts by the crystal, and therefore follows different paths through the crystal. The components of a divided light beam follow different paths within the mineral and travel at different speeds. When the mineral is observed at some a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage."Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica." Diamond and graphite provide examples of cleavage. Both are composed solely of a single element, carbon. But in diamond, each carbon atom is bonded to four others in a tetrahedral pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unit Cube
A unit cube, more formally a cube of side 1, is a cube whose sides are 1 unit long.. See in particulap. 671. The volume of a 3-dimensional unit cube is 1 cubic unit, and its total surface area is 6 square units.. Unit hypercube The term ''unit cube'' or unit hypercube is also used for hypercubes, or "cubes" in ''n''-dimensional spaces, for values of ''n'' other than 3 and edge length 1. Sometimes the term "unit cube" refers in specific to the set , 1sup>''n'' of all ''n''-tuples of numbers in the interval , 1 The length of the longest diagonal of a unit hypercube of ''n'' dimensions is \sqrt n, the square root of ''n'' and the (Euclidean) length of the vector (1,1,1,....1,1) in ''n''-dimensional space. See also *Doubling the cube * K-cell *Robbins constant, the average distance between two random points in a unit cube *Tychonoff cube, an infinite-dimensional analogue of the unit cube *Unit square *Unit sphere In mathematics, a unit sphere is simply a sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
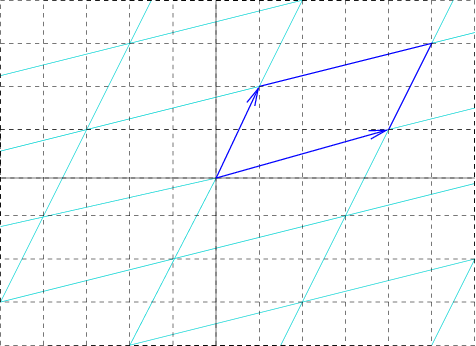
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinel
Spinel () is the magnesium/aluminium member of the larger spinel group of minerals. It has the formula in the cubic crystal system. Its name comes from the Latin word , which means ''spine'' in reference to its pointed crystals. Properties Spinel crystallizes in the isometric system; common crystal forms are octahedra, usually twinned. It has no true cleavage, but shows an octahedral parting and a conchoidal fracture. Its hardness is 8, its specific gravity is 3.5–4.1, and it is transparent to opaque with a vitreous to dull luster. It may be colorless, but is usually various shades of red, lavender, blue, green, brown, black, or yellow. Some spinels are among the most famous gemstones; among them are the Black Prince's Ruby and the "Timur ruby" in the British Crown Jewels, and the "Côte de Bretagne", formerly from the French Crown jewels. The Samarian Spinel is the largest known spinel in the world, weighing . The transparent red spinels were called spinel-rubies or b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word ''gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum (calcined gypsum) used for various purposes, this dehydrated gypsum became known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for Lime (material), lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeology, archaeologists and stone trade pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |