|
Mordant (other)
A mordant or dye fixative is a substance used to set (i.e., bind) dyes on fabrics. It does this by forming a coordination complex with the dye, which then attaches to the fabric (or tissue). It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. Although mordants are still used, especially by small batch dyers, they have been largely displaced in industry by directs.} The term mordant comes from the Latin ''mordere'', "to bite". In the past, it was thought that a mordant helped the dye "bite" onto the fiber so that it would hold fast during washing. A mordant is often a polyvalent metal ion, and one example is chromium (III). The resulting coordination complex of dye and ion is colloidal and can be either acidic or alkaline. Common dye mordants Mordants include tannic acid, oxalic acid, alum, chrome alum, sodium chloride, and certain salts of aluminium, chromium, copper, iron, iodine, potassium, sodium, tungsten, and tin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
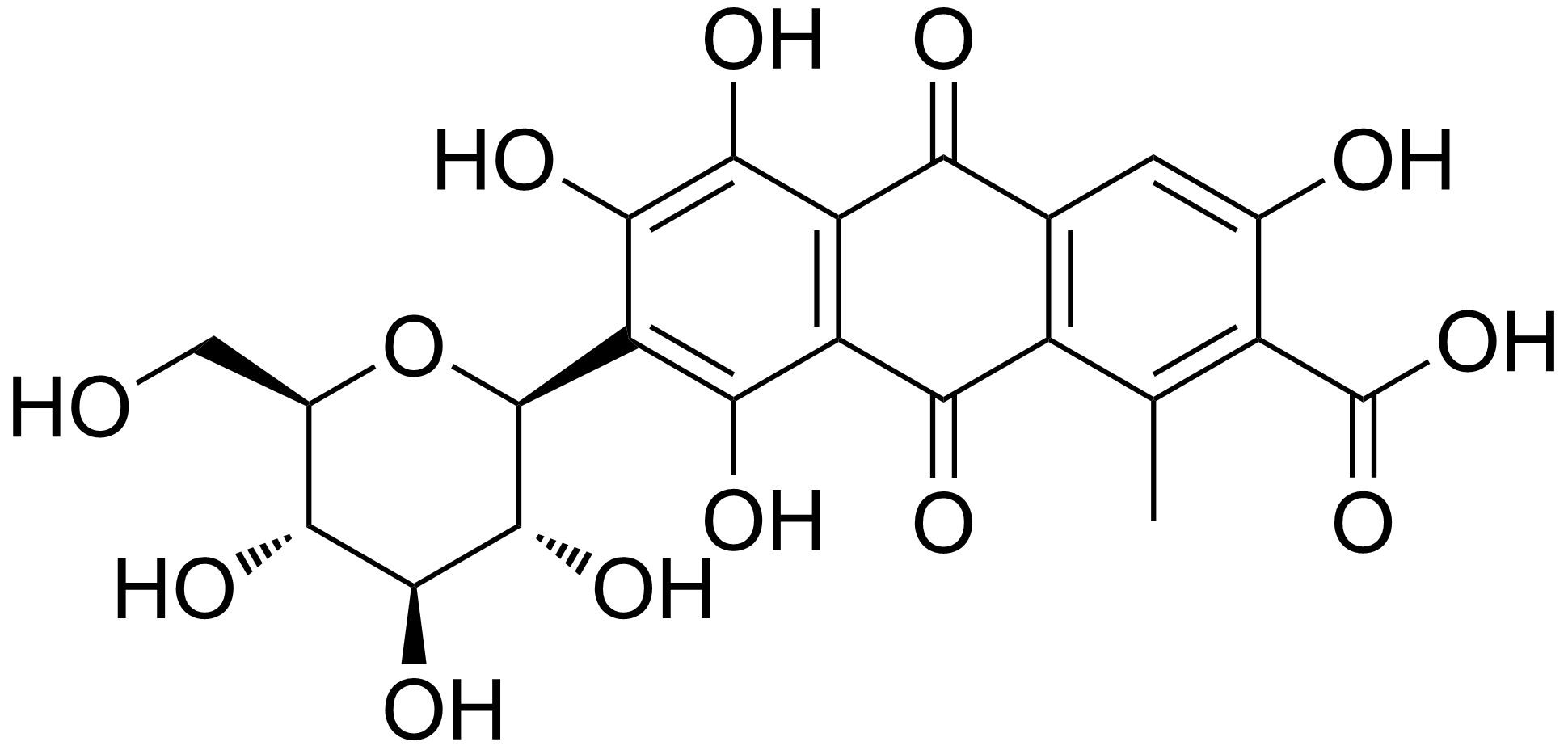
Mordant Red 19
Mordant red 19 is an organic compound with the chemical formula C16H13ClN4O5S. It is classified as an azo dye.Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel: "Azo Dyes" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim.. It is a mordant used in textile dyeing, usually in combination with chromium. It is usually found as the sodium salt. See also *Alizarin *List of colors These are the lists of colors; * List of colors: A–F * List of colors: G–M * List of colors: N–Z * List of colors (compact) * List of colors by shade * List of color palettes * List of Crayola crayon colors * List of RAL colors * List ... References External linksGoogle images of Mordant Red 19 Shades of red Azo dyes Pyrazolones Sulfonic acids Chloroarenes Phenols {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chrome Alum
Chrome alum or Chromium(III) potassium sulfate is the potassium double sulfate of chromium. Its chemical formula is KCr(SO4)2 and it is commonly found in its dodecahydrate form as KCr(SO4)2·12(H2O). It is used in leather tanning. Production and properties Chromium alum is produced from chromate salts or from ferrochromium alloys. Concentrated aqueous solutions of potassium dichromate can be reduced, usually with sulfur dioxide but also with alcohols or formaldehyde, in the presence of sulfuric acid at temperatures <40 °C. Alternatively and less commonly, ferrochromium alloys can be dissolved in sulfuric acid and, after precipitation of the ferrous sulfate, the chrome alum crystallizes upon addition of . Chromium alum crystallizes in regular |
Cochineal
The cochineal ( , ; ''Dactylopius coccus'') is a scale insect in the suborder Sternorrhyncha, from which the natural dye carmine is derived. A primarily sessility (motility), sessile parasitism, parasite native to tropical and subtropical South America through North America (Mexico and the Southwest United States), this insect lives on Cactus, cacti in the genus ''Opuntia'', feeding on plant moisture and nutrients. The insects are found on the pads of prickly pear cacti, collected by brushing them off the plants, and dried. The insect produces carminic acid that deters predation by other insects. Carminic acid, typically 17–24% of dried insects' weight, can be extracted from the body and eggs, then mixed with aluminium or calcium salts to make carmine dye, also known as cochineal. Today, carmine is primarily used as a Food coloring, colorant in food and in lipstick (Carmine, E120 or Carminic acid, Natural Red 4). Carmine dye was used in the Americas for coloring fabrics and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tints And Shades
In color theory, a tint is a mixture of a color with white, which increases lightness, while a shade is a mixture with black, which increases darkness. Both processes affect the resulting color mixture's relative saturation. A tone is produced either by mixing a color with gray, or by both tinting and shading. Mixing a color with any neutral color (including black, gray, and white) reduces the chroma, or colorfulness, while the hue (the relative mixture of red, green, blue, etc. depending on the colorspace) remains unchanged. In the graphic arts, especially printmaking and drawing, "tone" has a different meaning, referring to areas of continuous color, produced by various means, as opposed to the linear marks made by an engraved or drawn line. In common language, the term ''shade'' can be generalized to encompass any varieties of a particular color, whether technically they are shades, tints, tones, or slightly different hues. Meanwhile, the term ''tint'' can be generali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dye Rot
A dye is a wiktionary:colored, colored substance that chemically bonds to the wikt:substrate, substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber. There are two broad categories of dyes: Natural dye, natural and synthetic; Natural dyes are dyes extracted from Plant, plants, Insect, Insects, or Mineral, minerals. The majority of natural dyes are vegetable dyes derived from plant sources such as Root, roots, Berry, berries, Bark (botany), bark, Leaf, leaves, and wood, as well as other biological sources like Fungus, fungi. Synthetic dyes are also referred to as "coal tar dyes" because they are derived from Chemical substance, substances that, until recently, could only be extracted from coal tar. A synthetic dye consists of a chromophore and an auxochrome added to a benzene deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram Staining
In microbiology and bacteriology, Gram stain (Gram staining or Gram's method), is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique in 1884. Gram staining differentiates bacteria by the chemical and physical properties of their cell walls. Gram-positive cells have a thick layer of peptidoglycan in the cell wall that retains the primary stain, crystal violet. Gram-negative cells have a thinner peptidoglycan layer that allows the crystal violet to wash out on addition of ethanol. They are stained pink or red by the counterstain, commonly safranin or fuchsine. Lugol's iodine solution is always added after addition of crystal violet to strengthen the bonds of the stain with the cell membrane. Gram staining is almost always the first step in the preliminary identification of a bacterial organism. While Gram stain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_Potassium_Sulfate-crystals.jpg)