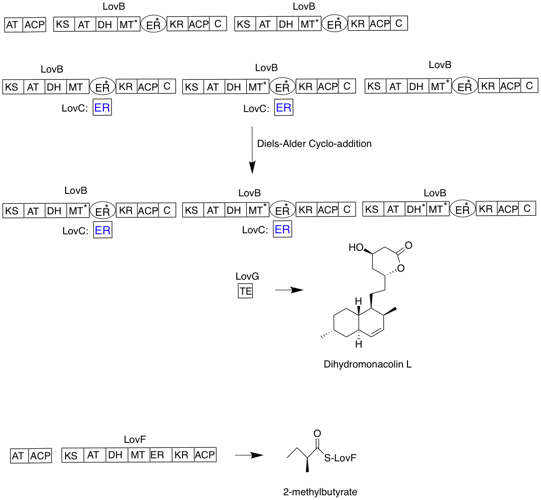
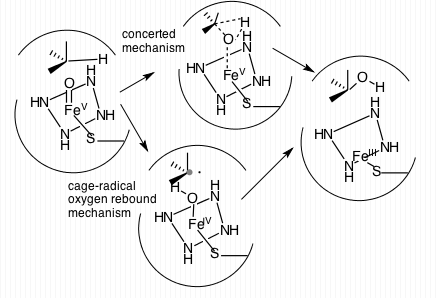
|
Mevacor
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth. Common side effects include diarrhea, constipation, headache, muscles pains, rash, and trouble sleeping. Serious side effects may include liver problems, muscle breakdown, and kidney failure. Use during pregnancy may harm the baby and use during breastfeeding is not recommended. It works by decreasing the liver's ability to produce cholesterol by blocking the enzyme HMG-CoA reductase. Lovastatin was patented in 1979 and approved for medical use in 1987. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 99th most commonly prescribed medication in the United States, with more than 7million prescriptions. Medical uses The primary uses of lovastatin is for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statin Medication
Statins, also known as HMG-CoA reductase inhibitors, are a Drug class, class of lipid-lowering medications that reduce illness and mortality in those who are at high risk of cardiovascular disease. They are the most common cholesterol-lowering drugs. Low-density lipoprotein (LDL) carriers of cholesterol play a key role in the development of atherosclerosis and coronary heart disease via the mechanisms described by the lipid hypothesis. Statins are effective in lowering LDL cholesterol and so are widely used for primary prevention in people at high risk of cardiovascular disease, as well as in secondary prevention for those who have developed cardiovascular disease. Side effects of statins include Myalgia, muscle pain, increased risk of diabetes mellitus, and abnormal blood levels of liver function tests, liver enzymes. Additionally, they have rare but severe adverse effects, particularly muscle damage. They Enzyme inhibitor, inhibit the enzyme HMG-CoA reductase which plays a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth. Common side effects include joint pain, diarrhea, heartburn, nausea, and muscle pains. Serious side effects may include rhabdomyolysis, liver problems, and diabetes. Use during pregnancy may harm the fetus. Like all statins, atorvastatin works by inhibiting HMG-CoA reductase, an enzyme found in the liver that plays a role in producing cholesterol. Atorvastatin was patented in 1986, and approved for medical use in the United States in 1996. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the most commonly prescribed medication in the United States, with more than 114million prescriptions. Medical uses The primary uses of atorvastatin is for the treatment of dyslipide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG-CoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simvastatin
Simvastatin, sold under the brand name Zocor among others, is a statin, a type of lipid-lowering medication. It is used along with exercise, diet, and weight loss to decrease elevated lipid levels. It is also used to decrease the risk of heart problems in those at high risk. It is taken by mouth. Common side effects include constipation, headaches, and nausea. Serious side effects may include muscle breakdown, liver problems, and increased blood sugar levels. A lower dose may be needed in people with kidney problems. There is evidence of harm to the developing baby when taken during pregnancy and it should not be used by those who are breastfeeding. It is in the statin class of medications and works by decreasing the manufacture of cholesterol by the liver. Simvastatin is made from the fungus ''Aspergillus terreus''. It was patented by Merck in 1980, and came into medical use in 1992. Simvastatin is available as a generic medication, and is on the World Health Organization' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
By Mouth
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dyspepsia
Indigestion, also known as dyspepsia or upset stomach, is a condition of impaired digestion. Symptoms may include upper abdominal fullness, heartburn, nausea, belching, or upper abdominal pain. People may also experience feeling full earlier than expected when eating. Indigestion is relatively common, affecting 20% of people at some point during their life, and is frequently caused by gastroesophageal reflux disease (GERD) or gastritis. Indigestion is subcategorized as "organic" or "functional", but making the diagnosis can prove challenging for physicians. Organic indigestion is the result of an underlying disease, such as gastritis, peptic ulcer disease (an ulcer of the stomach or duodenum), or cancer. Functional indigestion (previously called nonulcer dyspepsia) is indigestion without evidence of underlying disease. Functional indigestion is estimated to affect about 15% of the general population in western countries and accounts for a majority of dyspepsia cases. In elderl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furanocoumarin
The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. Most of the plant species found to contain furanocoumarins belong to a handful of plant families. The families Apiaceae and Rutaceae include the largest numbers of plant species that contain furanocoumarins. The families Moraceae and Fabaceae include a few widely distributed plant species that contain furanocoumarins. Generally furanocoumarins are most abundant in plants that have flowered and in ripe seeds and fruits. (An exception is the common fig where furanocoumarins are found chiefly in the milky sap of the leaves and shoots but not the fruits. Cited in McGovern and Barkley 2000, section&nbsPhytophotodermatitis) During the early stages of plant growth, their presence is not easily detected. Structure The chemical structure of furanocoumarins consists of a furan ring fused with a coumarin. The furan ring may be fused in various ways producing several differen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naringin
Naringin is a flavanone-7-''O''-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness created by naringin. In humans naringin is metabolized to the aglycone naringenin (not bitter) by naringinase present in the gut. Structure Naringin belongs to the flavonoid family. Flavonoids consist of 15 carbon atoms in 3 rings, 2 of which must be benzene rings connected by a 3 carbon chain. Naringin contains the basic flavonoid structure along with one rhamnose and one glucose unit attached to its aglycone portion, called naringenin, at the 7-carbon position. The steric hindrance provided by the two sugar units makes naringin less potent than its aglycone counterpart, naringenin. Metabolism In humans, naringinase is f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grapefruit
The grapefruit (''Citrus'' × ''paradisi'') is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The interior flesh is segmented and varies in color from pale yellow to dark pink. Grapefruit is a citrus hybrid originating in Barbados. It is an accidental cross between the sweet orange (''C. sinensis'') and the pomelo or shaddock (''C. maxima''), both of which were introduced from Asia in the 17th century. It has also been called the ''forbidden fruit''. In the past it was referred to as the ''pomelo'', but that term is now mostly used as the common name for ''Citrus maxima''. In 2019, world production of grapefruits (combined with pomelos) was 9.3 million tonnes, of which 53% was in China. Other significant producers include Vietnam, United States and Mexico. Description The evergreen grapefruit trees usually grow to around tall, although they may reach . The leaves are long (up to ), thin, glossy, and dark green. They produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs which are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will therefore either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein containing a heme group with an iron atom. In humans, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contraindication
In medicine, a contraindication is a condition that serves as a reason not to take a certain medical treatment due to the harm that it would cause the patient. Contraindication is the opposite of indication, which is a reason to use a certain treatment. ''Absolute contraindications'' are contraindications for which there are no reasonable circumstances for undertaking a course of action. For example, children and teenagers with viral infections should not be given aspirin because of the risk of Reye syndrome, and a person with an anaphylactic food allergy should never eat the food to which they are allergic. Similarly, a person with hemochromatosis should not be administered iron preparations. ''Relative contraindications'' are contraindications for circumstances in which the patient is at higher risk of complications from treatment, but these risks may be outweighed by other considerations or mitigated by other measures. For example, a pregnant woman should normally avoid gett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |