|
Meta-diethynylbenzene Dianion
In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula , three positional isomers are possible, differing in the relative positions of the two substituents around the ring: *''ortho''-diethynylbenzene dianion *''meta''-diethynylbenzene dianion *''para''-diethynylbenzene dianion The gaseous state of all three anions are of theoretical interest. They have been generated by decarboxylation of benzene di propynoic acids, using the technique of mass spectrometry. The three isomers of the dianion are the three strongest known superbases ever, with the ''ortho'' isomer being the strongest, with a proton affinity of . The ''meta'' isomer is the second-strongest, and the ''para'' isomer is the third-strongest. Observation These dianions were generated in a linear quadrupole ion-trap mass spectrometer. Electrospray ionization (ESI) of the diacid precursor results in the dicarboxylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Monoxide Anion
Lithium monoxide anion (LiO−) is a superbase existing in the gas phase. It was the strongest known base until 2008, when the isomeric diethynylbenzene dianions were determined to have a higher proton affinity. The methanide ion CH3− was the strongest known base before lithium monoxide anion was discovered. LiO− has a proton affinity of ~1782 kJ mol−1. Synthesis of the lithium monoxide anion The anion is prepared in a mass spectrometer by successive decarboxylation and decarbonylation of lithium oxalate anion under collision induced dissociation (CID) conditions: LiO(C=O)(CO) → LiOCO− + CO2, LiOCO− → LiO− + CO. The above method to synthesize the lithium monoxide anion is inefficient and difficult to carry out. The required ion rapidly reacts with traces of moisture and molecular oxygen present in the air. The reaction is further intensified by the high pressure argon that is introduced into the instrument to carry out the CID step. References See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Hydride Ion
The helium hydride ion or hydridohelium(1+) ion or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang. The ion was first produced in a laboratory in 1925. It is stable in isolation, but extremely reactive, and cannot be prepared in bulk, because it would react with any other molecule with which it came into contact. Noted as the strongest known acid—stronger than even fluoroantimonic acid—its occurrence in the interstellar medium had been conjectured since the 1970s, and it was finally detected in April 2019 using the airborne SOFIA telescope. Physical properties The helium hydrogen ion is isoelectronic with molecular hydrogen (). Unlike the dihydrogen ion , the helium hydride ion has a permanent dipole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
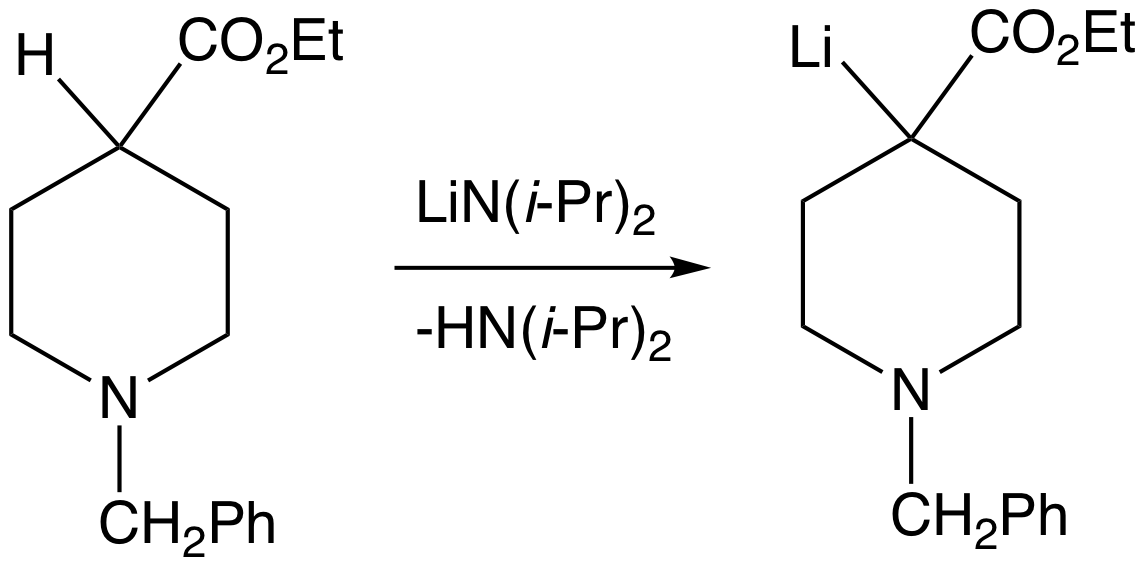
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other plane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collision-induced Dissociation
Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions (typically molecular ions or protonated molecules) are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules (often helium, nitrogen or argon). In the collision some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry. CID and the fragment ions produced by CID are used for several purposes. Partial or complete structural determination can be achieved. In some cases identity can be established based on previous knowledge without determining structure. Another use is in simply achieving more sensitive and specific detection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |