|
Meso Position
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * In ''ortho''-substitution, two substituents occupy positions next to each other, which may be numbered 1 and 2. In the diagram, these positions are marked R and ''ortho''. * In ''meta''-substitution the substituents occupy positions 1 and 3 (corresponding to R and ''meta'' in the diagram). * In ''para''-substitution, the substituents occupy the opposite ends (positions 1 and 4, corresponding to R and ''para'' in the diagram). The toluidines serve as an example for these three types of substitution. Synthesis Electron donating groups, for example amino, hydroxyl, alkyl, and phenyl groups tend to be ''ortho''/''para''-directors, and electron withdrawing groups such as nitro, nitrile, and ketone groups, tend to be ''meta''-directors. Propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cocrystal
Cocrystals are "solids that are crystalline single phase materials composed of two or more different molecular or ionic compounds generally in a stoichiometric ratio which are neither solvates nor simple salts." A broader definition is that cocrystals "consist of two or more components that form a unique crystalline structure having unique properties." Several subclassifications of cocrystals exist. Cocrystals can encompass many types of compounds, including hydrates, solvates and clathrates, which represent the basic principle of host–guest chemistry. Hundreds of examples of cocrystallization are reported annually. History The first reported cocrystal, quinhydrone, was studied by Friedrich Wöhler in 1844. Quinhydrone is a cocrystal of quinone and hydroquinone (known archaically as quinol). He found that this material was made up of a 1:1 molar combination of the components. Quinhydrone was analyzed by numerous groups over the next decade and several related cocrystals were m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryne
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes. Bonding in arynes The alkyne representation of benzyne is the most widely encountered. Arynes are usually described as having a strained triple bond. Geometric constraints on the triple bond in benzyne result in diminished overlap of in-plane p-orbitals, and thus weaker triple bond. The vibrational frequency of the triple bond in benzyne was assigned by Radziszewski to be 1846 cm−1, indicating a weaker triple bond than in unstrained alkyne with vibrational frequency of approximately 2150 cm−1. Nevertheless, benzyne is more like a strained alkyne than a diradical, as seen from the large singlet–triplet gap and alkyne-like reactivity. The LUMO of aryne lies much lower than the LUMO of un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
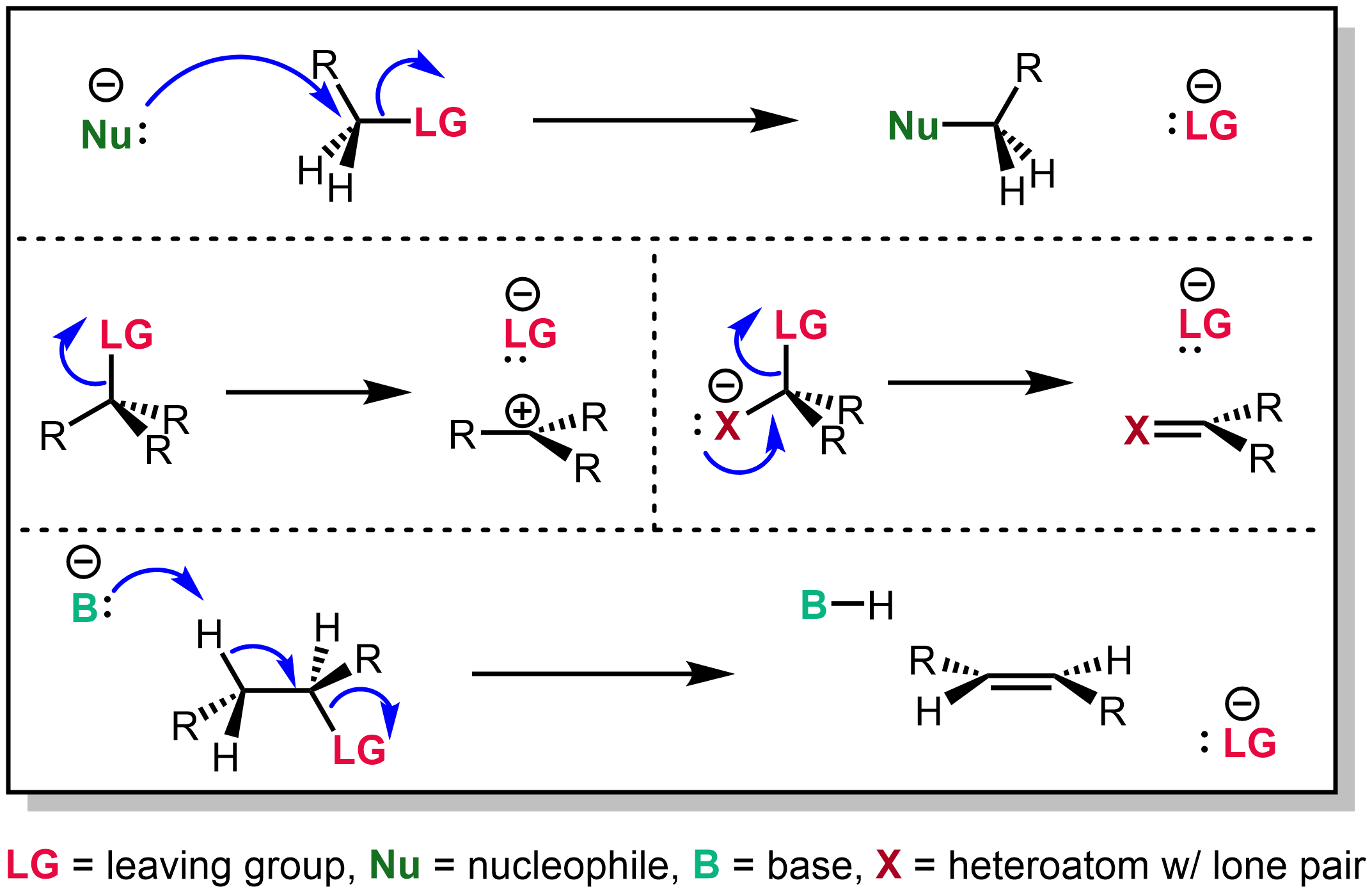
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term ''nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acridine
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound. Isolation and syntheses Carl Gräbe and Heinrich Caro first isolated acridine in 1870 from coal tar. Acridine is separated from coal tar by extracting with dilute sulfuric acid. Addition of potassium dichromate to this solution precipitates acridine bichromate. The bichromate is decomposed using ammonia. Acridine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry. Nomenclature Calixarene nomenclature is straightforward and involves counting the number of repeating units in the ring and including it in the name. A calix rene has 4 units in the ring and a calix rene has 6. A substituent in the meso position Rb is added to the name with a prefix C- as in C-methylcalix rene The word calixarene is derived from the Greek calix or chalice because this type of molecule resembles a vase (or cup) and from the word arene that refers to the aromatic building block. Synthesis Calixarenes are generally produced by condensation of two components: an electron-rich aromatic compound, classically a 4-substituted phenol, and an aldehyde, classically formaldehyde. *The scope for the aromatic component is broad diverse. The k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group. Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations The abbreviation "Bn" denotes be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way trimethylsiloxy groups minus;O-Si(CH3)3are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethylsilyl chloride and bis(trimethylsilyl)acetamide. Tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |