|
Mavacoxib
Mavacoxib (trade name Trocoxil) is a veterinary drug used to treat pain and inflammation in dogs with degenerative joint disease. It acts as a COX-2 inhibitor. Mavacoxib, along with other COX-2 selective inhibitors, celecoxib, valdecoxib, and parecoxib, were discovered by a team at the Searle division of Monsanto The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best known product is Roundup, a glyphosate-based herbicide, developed in th ... led by John Talley. References COX-2 inhibitors Nonsteroidal anti-inflammatory drugs Fluoroarenes Sulfonamides Pyrazoles Trifluoromethyl compounds {{musculoskeletal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Veterinary Drug
An animal drug (also veterinary drug) refers to a drug intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in animals. Regulation United States The U.S. Food and Drug Administration (FDA) has the broad mandate under the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 321 et seq.) to assure the safety and effectiveness of animal drugs and their use in all animals, including farm animals. The division of the FDA responsible for this is the Center for Veterinary Medicine (CVM). The equivalents of the Investigational New Drug and New Drug Application are known as the Investigational New Animal Drug and New Animal Drug Application, respectively. Before CVM formally approves an animal drug, the sponsor or manufacturer of the drug must document in scientific testing that the drug has been found "safe and effective". The testing data also must demonstrate that a methodology is available to detect and measure any residue left in edible animal produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COX-2 Inhibitor
COX-2 inhibitors are a type of nonsteroidal anti-inflammatory drug (NSAID) that directly targets cyclooxygenase-2, COX-2, an enzyme responsible for inflammation and pain. Targeting selectivity for COX-2 reduces the risk of peptic ulceration and is the main feature of celecoxib, rofecoxib, and other members of this drug class. After several COX-2-inhibiting drugs were approved for marketing, data from clinical trials revealed that COX-2 inhibitors caused a significant increase in heart attacks and strokes, with some drugs in the class having worse risks than others. Rofecoxib (sold under the brand name Vioxx) was taken off the market in 2004 because of these concerns, while celecoxib (sold under the brand name Celebrex) and traditional NSAIDs received boxed warnings on their labels. Many COX-2-specific inhibitors have been removed from the US market. As of December 2011, only Celebrex (generic name of celecoxib) is still available for purchase in the United States. In the European ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib was patented in 1993 and came into medical use in 1999 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valdecoxib
Valdecoxib is a nonsteroidal anti-inflammatory drug (NSAID) used in the treatment of osteoarthritis, rheumatoid arthritis, and painful menstruation and menstrual symptoms. It is a selective cyclooxygenase-2 inhibitor. It was patented in 1995. Valdecoxib was manufactured and marketed under the brand name Bextra by G. D. Searle & Company as an anti-inflammatory arthritis drug. It was approved by the United States Food and Drug Administration on November 20, 2001, to treat arthritis and menstrual cramps. and was available by prescription in tablet form until 2005 when the FDA requested that Pfizer withdraw Bextra from the American market. The FDA cited "potential increased risk for serious cardiovascular (CV) adverse events," an "increased risk of serious skin reactions" and the "fact that Bextra has not been shown to offer any unique advantages over the other available NSAIDs." In 2009 Bextra was at the center of the "largest health care fraud settlement and the largest criminal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parecoxib
Parecoxib, sold under the brand name Dynastat among others, is a water-soluble and injectable prodrug of valdecoxib. Parecoxib is a COX2 selective inhibitor. It is injectable. It is approved through much of Europe for short term perioperative pain control. It was patented in 1996 and approved for medical use in 2002. Approval In 2005, the U.S. Food and Drug Administration (FDA) issued a letter of non-approval for parecoxib in the United States. No reasons were ever documented publicly for the non-approval, although one study noted increased occurrences of heart attacks following cardiac bypass surgery compared to placebo when high doses of parecoxib were used to control pain after surgery. Importantly, rare but severe allergic reactions (Stevens–Johnson syndrome, Lyell syndrome) have been described with valdecoxib, the molecule to which parecoxib is converted. The drug is not approved for use after cardiac surgery in Europe. All anti-inflammatory medications in the U.S. ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monsanto
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best known product is Roundup, a glyphosate-based herbicide, developed in the 1970s. Later the company became a major producer of genetically engineered crops. In 2018, the company ranked 199th on the Fortune 500 of the largest United States corporations by revenue. Monsanto was one of four groups to introduce genes into plants in 1983, and was among the first to conduct field trials of genetically modified crops in 1987. It was one of the top 10 US chemical companies until it divested most of its chemical businesses between 1997 and 2002, through a process of mergers and spin-offs that focused the company on biotechnology. Monsanto was one of the first companies to apply the biotechnology industry business model to agriculture, using techniques developed by biotech drug companies. In this business model, compani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Talley (chemist)
John J. Talley is an American medicinal chemist who was the lead chemist in the discovery of the COX-2 selective nonsteroidal anti-inflammatory drug celecoxib and a co-inventor of amprenavir, a protease inhibitor used to treat HIV infection. He earned his BA at the University of Northern Iowa and his PhD in chemistry from the University of Minnesota. From 1979 to 1986 he worked at the General Electric R&D center in Schenectady, New York then joined the Searle division of Monsanto, where he led the team that discovered celecoxib, as well as other marketed COX-2 inhibitors valdecoxib, parecoxib, and mavacoxib, as well as amprenavir which licensed by Searle to Vertex Pharmaceuticals Vertex Pharmaceuticals is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headqua .... In 2002 he was hired by Microbia to lead their a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COX-2 Inhibitors
COX-2 inhibitors are a type of nonsteroidal anti-inflammatory drug (NSAID) that directly targets cyclooxygenase-2, COX-2, an enzyme responsible for inflammation and pain. Targeting selectivity for COX-2 reduces the risk of peptic ulceration and is the main feature of celecoxib, rofecoxib, and other members of this drug class. After several COX-2-inhibiting drugs were approved for marketing, data from clinical trials revealed that COX-2 inhibitors caused a significant increase in heart attacks and strokes, with some drugs in the class having worse risks than others. Rofecoxib (sold under the brand name Vioxx) was taken off the market in 2004 because of these concerns, while celecoxib (sold under the brand name Celebrex) and traditional NSAIDs received boxed warnings on their labels. Many COX-2-specific inhibitors have been removed from the US market. As of December 2011, only Celebrex (generic name of celecoxib) is still available for purchase in the United States. In the European ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. Most NSAI ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
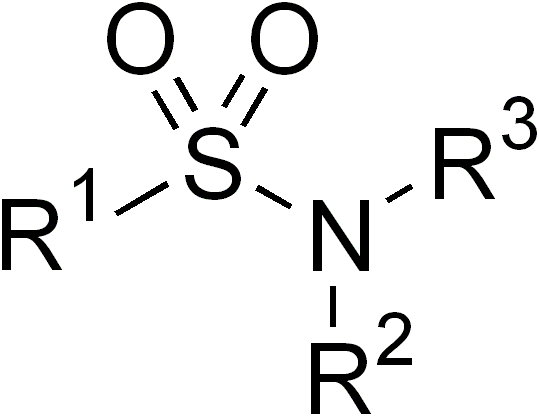
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |