|
MYOM1
Myomesin-1 is a protein that in humans is encoded by the ''MYOM1'' gene. Myomesin-1 is expressed in muscle cells and functions to stabilize the three-dimensional conformation of the thick filament. Embryonic forms of Myomesin-1 have been detected in dilated cardiomyopathy. Structure Alternatively spliced variants of ''MYOM1'', including EH-myomesin, Skelemin and Myomesin-1 have been identified; with Skelemin having an additional 96 amino acids rich in serine and proline residues. Myomesin-1, like myomesin 2 and titin, is a member of a family of myosin-associated proteins containing structural modules with strong homology to either fibronectin type III (motif I) or immunoglobulin C2 (motif II) domains. Myomesin-1 bears uniqueness within this family in that it has intermediate filament core-like motifs, one near each terminus. Myomesin-1 and Myomesin-2 each have a unique N-terminal region followed by 12 modules of motif I or motif II, in the arrangement II-II-I-I-I-I-I-II ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYOM2
Myomesin-2, also known as M-protein is a protein that in humans is encoded by the ''MYOM2'' gene. M-protein is expressed in adult cardiac muscle and fast skeletal muscle, and functions to stabilize the three-dimensional arrangement of proteins comprising M-band structures in a sarcomere. Structure Human M-protein is 165.0 kDa and 1465 amino acids in length. ''MYOM2'' is localized to the human chromosome 8p23.3. M-protein belong to the superfamily of cytoskeletal proteins having immunoglobulin/fibronectin repeats; M-protein contains two immunoglobulin C2-type repeats in the N-terminal region, five fibronectin type III repeats in the central region, and an additional four immunoglobulin C2-type repeats in the C-terminal region. M-protein is expressed only in striated muscle, including fast skeletal muscle and cardiac muscle. Function M-protein exhibits a different pattern of expression in cardiac and skeletal muscle, as well as fast- versus slow-skeletal muscle during development ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
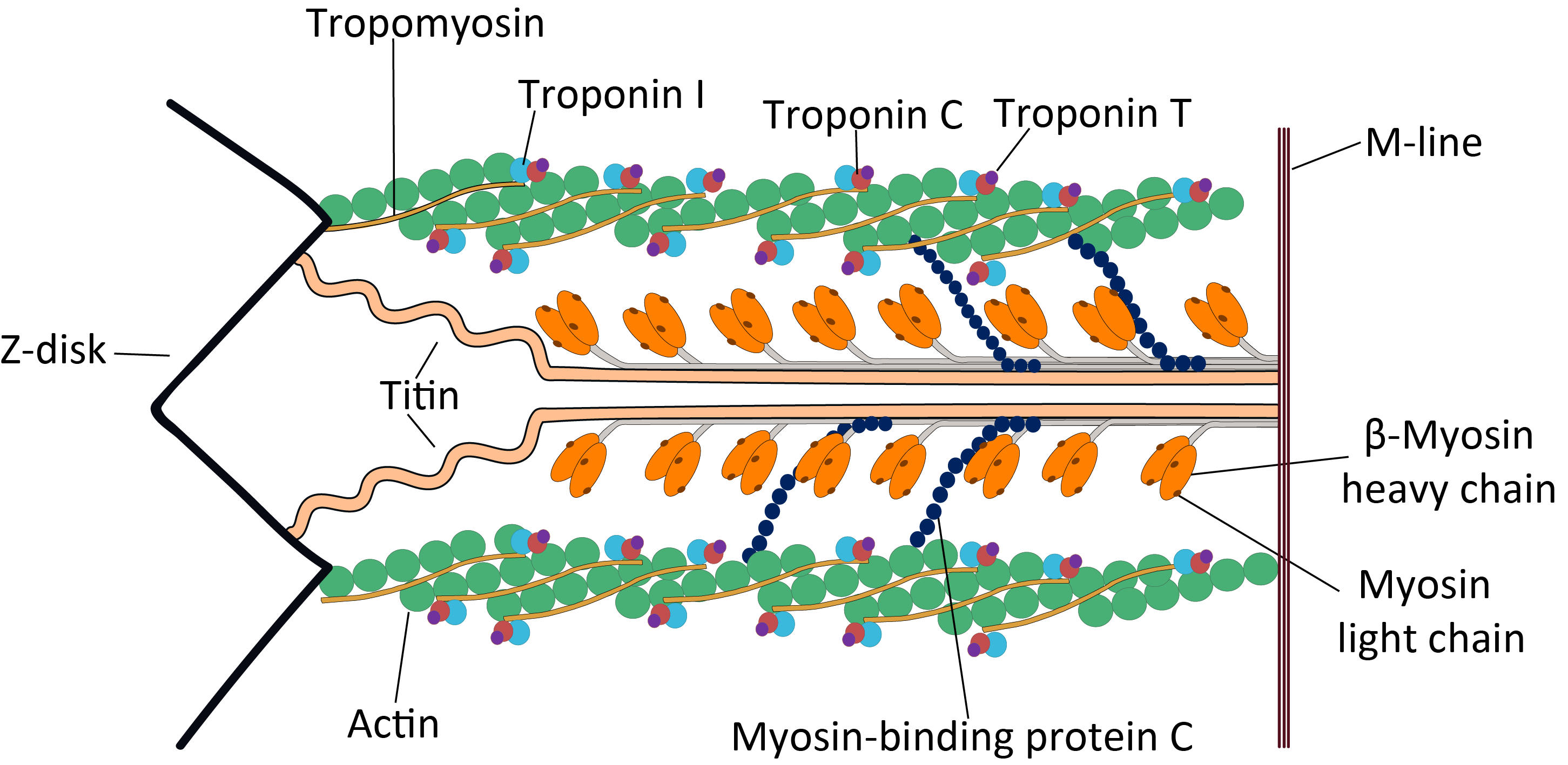
Titin
Titin (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z line to the M line in the sarcomere. The protein contributes to force transmission at the Z line and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titin
Titin (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z line to the M line in the sarcomere. The protein contributes to force transmission at the Z line and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibronectin Type III Domain
The Fibronectin type III domain is an evolutionarily conserved protein domain that is widely found in animal proteins. The fibronectin protein in which this domain was first identified contains 16 copies of this domain. The domain is about 100 amino acids long and possesses a beta sandwich structure. Of the three fibronectin-type domains, type III is the only one without disulfide bonding present. Fibronectin domains are found in a wide variety of extracellular proteins. They are widely distributed in animal species, but also found sporadically in yeast, plant and bacterial proteins. Human proteins containing this domain ABI3BP; ANKFN1; ASTN2; AXL; BOC; BZRAP1; C20orf75; CDON; CHL1; CMYA5; CNTFR; CNTN1; CNTN2; CNTN3; CNTN4; CNTN5; CNTN6; COL12A1; COL14A1; COL20A1; COL7A1; CRLF1; CRLF3; CSF2RB; CSF3R; DCC; DSCAM; DSCAML1; EBI3; EGFLAM; EPHA1; EPHA10; EPHA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Domain
The immunoglobulin domain, also known as the immunoglobulin fold, is a type of protein domain that consists of a 2-layer sandwich of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek key topology, consisting of about 125 amino acids. The backbone switches repeatedly between the two β-sheets. Typically, the pattern is (N-terminal β-hairpin in sheet 1)-(β-hairpin in sheet 2)-(β-strand in sheet 1)-(C-terminal β-hairpin in sheet 2). The cross-overs between sheets form an "X", so that the N- and C-terminal hairpins are facing each other. Members of the immunoglobulin superfamily are found in hundreds of proteins of different functions. Examples include antibodies, the giant muscle kinase titin, and receptor tyrosine kinases. Immunoglobulin-like domains may be involved in protein–protein and protein–ligand interactions. Examples Human genes encoding proteins containing the immunoglobulin domain include: * A1BG * ACAM * ADAMTSL1 * ADAMTSL3 * AGER * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITGA2B
Integrin alpha-IIb is a protein that in humans is encoded by the ''ITGA2B'' gene. ITGA2B, also known as CD41, encodes integrin alpha chain 2b. Integrins are heterodimeric integral membrane proteins composed of an alpha chain and a beta chain. Alpha chain 2b undergoes post-translational cleavage to yield disulfide-linked light and heavy chains that join with beta 3 to form a fibrinogen receptor expressed in platelets that plays a crucial role in coagulation. Mutations that interfere with this role result in thrombasthenia. At least 38 disease-causing mutations in this gene have been discovered. In addition to adhesion, integrins are known to participate in cell-surface mediated signalling. Interactions ITGA2B has been shown to interact with AUP1 and CLNS1A. See also * Cluster of differentiation * Glycoprotein IIb/IIIa In medicine, glycoprotein IIb/IIIa (GPIIb/IIIa, also known as integrin αIIbβ3) is an integrin complex found on platelets. It is a receptor for fibrinogen and vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITGB3
Integrin beta-3 (β3) or CD61 is a protein that in humans is encoded by the ''ITGB3'' gene. CD61 is a cluster of differentiation found on thrombocytes. Structure and function The ITGB3 protein product is the integrin beta chain beta 3. Integrins are integral cell-surface proteins composed of an alpha chain and a beta chain. A given chain may combine with multiple partners resulting in different integrins. Integrin beta 3 is found along with the alpha IIb chain in platelets. Integrins are known to participate in cell adhesion as well as cell-surface-mediated signaling. Role in endometriosis Defectively expressed β3 integrin subunit has been correlated with presence of endometriosis, and has been suggested as a putative marker of this condition. Interactions CD61 has been shown to interact with PTK2, ITGB3BP, TLN1 and CIB1. See also * Glycoprotein IIb/IIIa In medicine, glycoprotein IIb/IIIa (GPIIb/IIIa, also known as integrin αIIbβ3) is an integrin complex found on platele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITGB1
Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ''ITGB1'' gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens. Integrin beta 1 is expressed as at least four different isoforms. In cardiac muscle and skeletal muscle, the integrin beta-1D isoform is specifically expressed, and localizes to costameres, where it aids in the lateral force transmission from the Z-discs to the extracellular matrix. Abnormal levels of integrin beta-1D have been found in limb girdle muscular dystrophy and polyneuropathy. Structure Integrin beta-1 can exist as different isoforms via alternative splicing. Six alternatively spliced variants have been found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myotonic Dystrophy
Myotonic dystrophy (DM) is a type of muscular dystrophy, a group of genetic disorders that cause progressive muscle loss and weakness. In DM, muscles are often unable to relax after contraction. Other manifestations may include cataracts, intellectual disability and heart conduction problems. In men, there may be early balding and an inability to have children. While myotonic dystrophy can occur at any age, onset is typically in the 20s and 30s. Myotonic dystrophy is caused by a genetic mutation in one of two genes. Mutation of the '' DMPK'' gene causes myotonic dystrophy type 1 (DM1). Mutation of '' CNBP'' gene causes type 2 (DM2). DM is typically inherited from a person's parents, following an autosomal dominant inheritance pattern, and it generally worsens with each generation. A type of DM1 may be apparent at birth. DM2 is generally milder. Diagnosis is confirmed by genetic testing. There is no cure. Treatments may include braces or wheelchairs, pacemakers and non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |