|
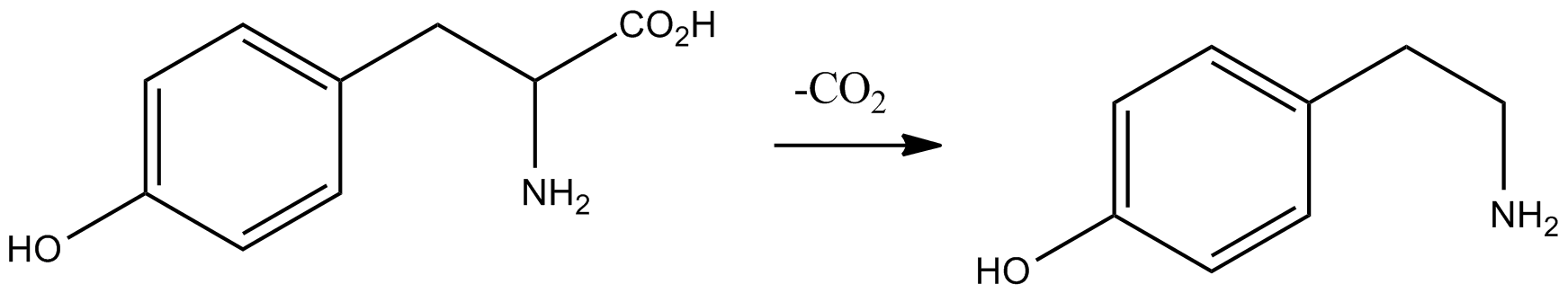
M-tyramine
''meta''-Tyramine, also known as ''m''-tyramine and 3-tyramine, is an endogenous trace amine neuromodulator and a structural analog of phenethylamine. It is a positional isomer of ''para''-tyramine, and similarly to it, has effects on the adrenergic and dopaminergic systems. ''meta''-Tyramine is produced in humans via aromatic amino acid decarboxylase-mediated metabolism of ''meta''-tyrosine. meta-Tyramine can be metabolized into dopamine via peripheral or brain CYP2D6 enzymes in humans. See also * ''para''-Tyramine * 3-Methoxytyramine 3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine that occurs as a metabolite of the neurotransmitter dopamine. It is formed by the introduction of a methyl group to dopamine by the enzyme catecho ... References Phenethylamines Phenols TAAR1 agonists Trace amines Norepinephrine-dopamine releasing agents {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of CYP2D6 enzyme produced betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor (chemistry), precursor chemical, L-DOPA, which is biosynthesis, synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopaminergic pathway, dopamine pathways, one of which plays a major role in the motivational component of reward system, reward-motivated behavior. The anticipa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogenous
Endogenous substances and processes are those that originate from within a living system such as an organism, tissue, or cell. In contrast, exogenous substances and processes are those that originate from outside of an organism. For example, estradiol is an endogenous estrogen hormone produced within the body, whereas ethinylestradiol Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disord ... is an exogenous synthetic estrogen, commonly used in birth control pills. References External links *{{Wiktionary-inline, endogeny Biology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TAAR1 Agonists
Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the ''TAAR1'' gene. TAAR1 is an intracellular amine-activated and G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells (e.g., the stomach, small intestine, duodenum, and white blood cells), astrocytes, and in the intracellular milieu within the presynaptic plasma membrane (i.e., axon terminal) of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski ''et al.'' and Bunzow ''et al.'' TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamines
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl- 2,5-dimethoxy-4-methylamphetamine DOM), entactogens (e.g., 3,4-methylenedioxyamphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Methoxytyramine
3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine that occurs as a metabolite of the neurotransmitter dopamine. It is formed by the introduction of a methyl group to dopamine by the enzyme catechol-O-methyl transferase (COMT). 3-MT can be further metabolized by the enzyme monoamine oxidase (MAO) to form homovanillic acid (HVA), which is then typically excreted in the urine. Originally thought to be physiologically inactive, 3-MT has recently been shown to act as an agonist of human TAAR1. Occurrence 3-Methoxytyramine occurs naturally in the prickly pear cactus (genus ''Opuntia''), and is in general widespread throughout the Cactaceae. It has also been found in crown gall tumors on ''Nicotiana'' sp. In humans, 3-methoxytyramine is a trace amine that occurs as a metabolite of dopamine. See also * Tyramine Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meta-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning '' cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with . They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and prevent reuptak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |