|
Ladderane
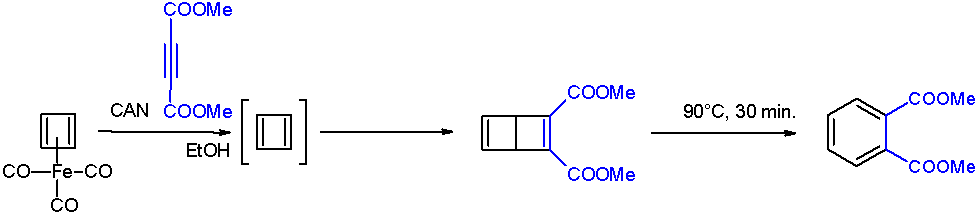
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the synthesis of ladderane compounds of various lengths. The mechanisms often involve + 2photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, phylum ''Planctomycetota''. Nomenclature Chain length Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1. A ladderane of 3 or more units can connect in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ladderane Nomenclature
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the Organic synthesis, synthesis of ladderane compounds of various lengths. The reaction mechanism, mechanisms often involve Woodward-Hoffmann rules, [2 + 2] photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, phylum ''Planctomycetota''. Nomenclature Chain length Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ladderane Stereochemistry
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the synthesis of ladderane compounds of various lengths. The mechanisms often involve + 2photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, phylum ''Planctomycetota''. Nomenclature Chain length Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe ladderanes based on the number of consecutive rings. The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (''n'') plus 1. A ladderane of 3 or more units can connect in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anammox
Anammox, an abbreviation for anaerobic ammonium oxidation, is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water. The bacteria that perform the anammox process are genera that belong to the bacterial phylum Planctomycetota. The anammox bacteria all possess one anammoxosome, a lipid bilayer membrane-bound compartment inside the cytoplasm in which the anammox process takes place. The anammoxosome membranes are rich in ladderane lipids; the presence of these lipids is so far unique in biology. "Anammox" is also the trademarked name for an anammox-based ammonium removal technology developedJetten Michael Silvester Maria, Van Loosdrecht Marinus Corneli; Technische Universiteit Delftpatent WO9807664/ref> by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planctomycetota
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammonium oxidation, also known as anammox. Many Planctomycetota occur in relatively high abundance as biofilms, often associating with other organisms such as macroalgae and marine sponges. Planctomycetota are included in the PVC superphylum along with Verrucomicrobiota, Chlamydiota, Lentisphaerota, Kiritimatiellaeota, and ''Candidatus'' ''Omnitrophica''. The phylum Planctomycetota is composed of the classes Planctomycetia and Phycisphaerae. First described in 1924, members of the Planctomycetota were identified as eukaryotes and were only later described as bacteria in 1972. Early examination of members of the Planctomycetota suggested a cell plan differing considerably from other bacteria, although they are now confirmed as Gram-negative bacte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prismanes
The prismanes are a class of hydrocarbon compounds consisting of prism-like polyhedra of various numbers of sides on the polygonal base. Chemically, it is a series of fused cyclobutane rings (a ladderane, with all-cis/all- syn geometry) that wraps around to join its ends and form a band, with cycloalkane edges. Their chemical formula is (C2H2)n, where ''n'' is the number of cyclobutane sides (the size of the cycloalkane base), and that number also forms the basis for a system of nomenclature within this class. The first few chemicals in this class are: Triprismane, tetraprismane, and pentaprismane have been synthesized and studied experimentally, and many higher members of the series have been studied using computer models. The first several members do indeed have the geometry of a regular prism, with flat ''n''-gon bases. As ''n'' becomes increasingly large, however, modeling experiments find that highly symmetric geometry is no longer stable, and the molecule distorts into les ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Amalgam
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium amalgams are often used in reactions as strong reducing agents with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension. Sodium amalgam was used as a reagent as early as 1862. A synthesis method was described by J. Alfred Wanklyn in 1866. Structure and compositions No particular formula is assigned to "sodium amalgam". Na5Hg8 and Na3Hg are well defined compounds. In sodium amalgams, the Hg-Hg distances are expanded to around 5 Å vs. about 3 Å for mercury itself. Usually amalgams are classified on the weight percent of sodium. Amalgams with 2% Na are solids at room temperature, whereas some more dilute amalgams remain liquid. Preparation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_Ladderane.png)