|
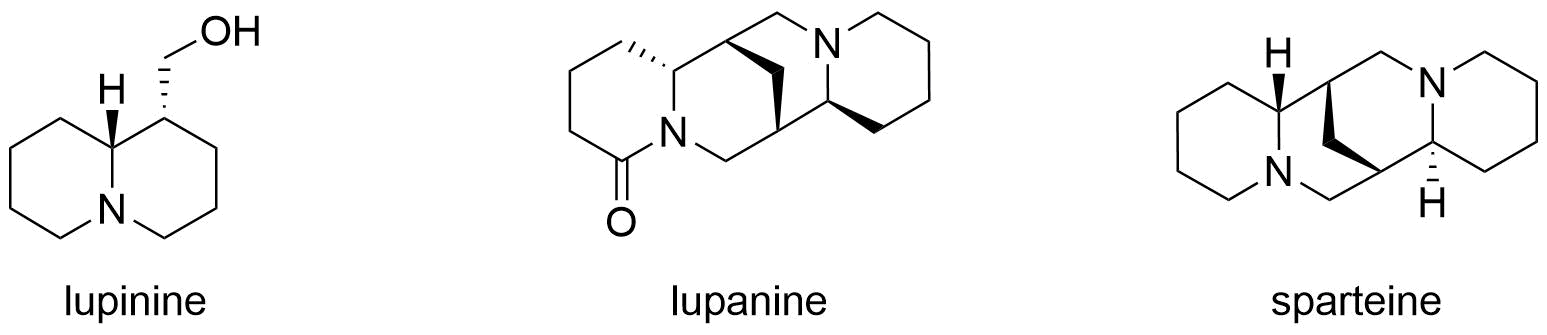
Lupinine
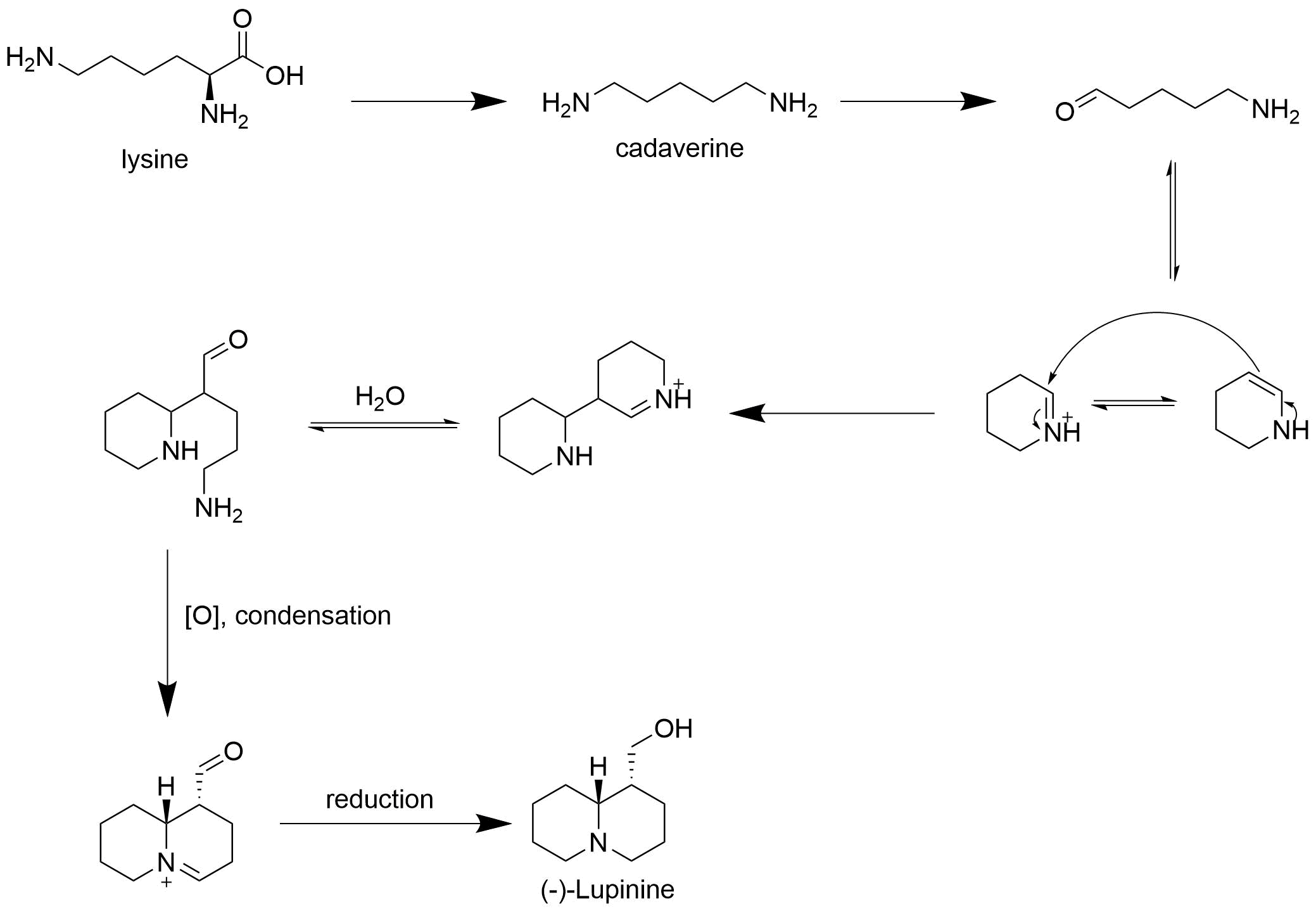
Lupinine is a quinolizidine alkaloid present in the genus ''Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinine Graphic 2
Lupinine is a quinolizidine alkaloid present in the genus '' Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinine Graphic 1
Lupinine is a quinolizidine alkaloid present in the genus '' Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinine Graphic 5
Lupinine is a quinolizidine alkaloid present in the genus '' Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolizidine Alkaloids
Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids. Occurrence Quinolizidine alkaloids can be found in the plant family of legumes, especially in papilionaceous plants. While the lupine alkaloids (following their name) can be found in lupines, tinctorin, for example, was isolated from the dyer's broom. Examples More than 200 quinolizidine alkaloids are known which can be classified into 6 structural types: * the lupinine type with 34 known structures, including lupinine and its derivatives * the camoensine type with 6 known structures, including camoensin * the spartein type with 66 structures, including sparteine, lupanine, angustifoline * the α-pyridone type with 25 structures, including anagyrine and cytisine * the matrine type with 31 structures, including matrine * and the ormosanin type with 19 structures, including ormosanine. (–)-Lupinine Structural Formula V2.svg, (–)-lupinine (6R,7S,9S,1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , Medicinal plant, plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogeny (biology)
Endogenous substances and processes are those that originate from within a living system such as an organism, tissue, or cell. In contrast, exogenous substances and processes are those that originate from outside of an organism. For example, estradiol is an endogenous estrogen hormone produced within the body, whereas ethinylestradiol Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disord ... is an exogenous synthetic estrogen, commonly used in birth control pills. References External links *{{Wiktionary-inline, endogeny Biology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolizidine
Quinolizidine (norlupinane, octahydro-2''H''-quinolizine) is a nitrogen-containing heterocyclic compound. Some alkaloids (e.g. cytisine and sparteine) are derivatives of quinolizidine. Quinolizidine alkaloids Quinolizidine alkaloids, such as nupharine and related chemicals, can be found in ''Nymphaea lotus'' and other species in the family Nymphaeaceae. Quinolizidine alkaloids are also found in genistoid legumes A legume () is a plant in the family Fabaceae (or Leguminosae), or the fruit or seed of such a plant. When used as a dry grain, the seed is also called a pulse. Legumes are grown agriculturally, primarily for human consumption, for livestock fo .... External links * * Synthesis: {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
IC50
The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance (e.g. drug) is needed to inhibit, ''in vitro'', a given biological process or biological component by 50%. The biological component could be an enzyme, cell, cell receptor or microorganism. IC50 values are typically expressed as molar concentration. IC50 is commonly used as a measure of antagonist drug potency in pharmacological research. IC50 is comparable to other measures of potency, such as EC50 for excitatory drugs. EC50 represents the dose or plasma concentration required for obtaining 50% of a maximum effect ''in vivo''. IC50 can be determined with functional assays or with competition binding assays. Sometimes, IC50 values are converted to the pIC50 scale. :\ce = -\log_ \ce Due to the minus sign, higher values of pIC50 indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotransmission
Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), and bind to and react with the receptors on the dendrites of another neuron (the postsynaptic neuron) a short distance away. A similar process occurs in retrograde neurotransmission, where the dendrites of the postsynaptic neuron release retrograde neurotransmitters (e.g., endocannabinoids; synthesized in response to a rise in intracellular calcium levels) that signal through receptors that are located on the axon terminal of the presynaptic neuron, mainly at GABAergic and glutamatergic synapses. Neurotransmission is regulated by several different factors: the availability and rate-of-synthesis of the neurotransmitter, the release of that neurotransmitter, the baseline activity of the postsynaptic cell, the number of available postsynapti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |