Lupinine on:
[Wikipedia]
[Google]
[Amazon]
Lupinine is a quinolizidine alkaloid present in the genus ''
lupinosis
which is a morbid, and often fatal condition that results in acute atrophy of liver function and which affects domestic animals such as cattle and sheep. When ingested by humans, quinolizidine alkaloid poisoning causes trembling, shaking, excitation, as well as convulsions. Lupinine, in addition to being orally toxic to mammals, is also an insect antifeedant as well as a growth inhibitor for the grasshopper.
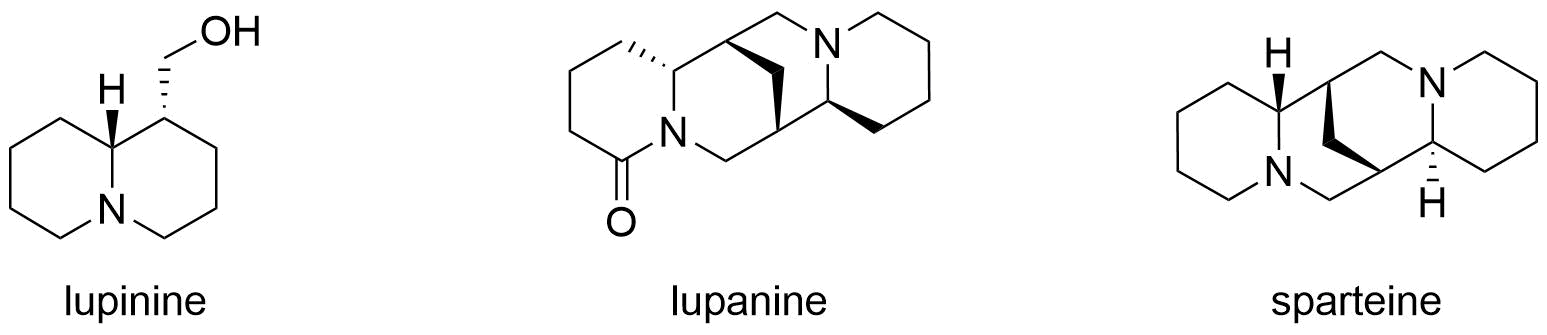 Lupinine, in comparison to other quinolizidine alkaloids commonly found in lupins, such as lupanine and
Lupinine, in comparison to other quinolizidine alkaloids commonly found in lupins, such as lupanine and
 Studies on the hydrochloride of lupinine have shown it to be a reversible inhibitor of acetylcholinesterases. Lupinine, a nitrogen-containing
Studies on the hydrochloride of lupinine have shown it to be a reversible inhibitor of acetylcholinesterases. Lupinine, a nitrogen-containing


Lupinus
''Lupinus'', commonly known as lupin, lupine, or regionally bluebonnet etc., is a genus of plants in the legume family Fabaceae. The genus includes over 199 species, with centers of diversity in North and South America. Smaller centers occur ...
'' (colloquially referred to as lupins
''Lupinus'', commonly known as lupin, lupine, or regionally bluebonnet etc., is a genus of plants in the legume family Fabaceae. The genus includes over 199 species, with centers of diversity in North and South America. Smaller centers occur ...
) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and ...
and that lupinine has an inhibitory effect on acetylcholine receptor
An acetylcholine receptor (abbreviated AChR) is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.
Classification
Like other transmembrane receptors, acetylcholine receptors are classified according ...
s. The characteristically bitter taste of lupin bean
Lupin or lupini beans are the yellow legume seeds of the genus ''Lupinus''. They are traditionally eaten as a pickled snack food, primarily in the Mediterranean basin ('' L. albus''), Latin America ('' L. mutabilis'') and North Africa ('' L. ang ...
s, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''.
Toxicity
Lupinine is ahepatotoxin
A hepatotoxin ('' Gr., hepato = liver'') is a toxic chemical substance that damages the liver.
It can be a side-effect, but hepatotoxins are also found naturally, such as microcystins and pyrrolizidine alkaloids, or in laboratory environments, su ...
prevalent in the seeds of leguminous herbs of the genus ''Lupinus''. Lupinine and other quinolizidine alkaloids give a bitter taste to naturally growing lupin flowers. Due to the toxicity of quinolizidine alkaloids, lupin beans are soaked overnight and rinsed to remove some of their alkaloid content. However, when the cooking and rinsing procedure is insufficient, 10 grams of seeds are able to liberate as much as 100 milligrams of lupinine.
The neurotoxicity
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specificall ...
of lupinine has been known within veterinary medical circles for some time due to the use of lupins as a forage feed for grazing livestock since it has high protein content. It is found to produclupinosis
which is a morbid, and often fatal condition that results in acute atrophy of liver function and which affects domestic animals such as cattle and sheep. When ingested by humans, quinolizidine alkaloid poisoning causes trembling, shaking, excitation, as well as convulsions. Lupinine, in addition to being orally toxic to mammals, is also an insect antifeedant as well as a growth inhibitor for the grasshopper.
Relative toxicity
 Lupinine, in comparison to other quinolizidine alkaloids commonly found in lupins, such as lupanine and
Lupinine, in comparison to other quinolizidine alkaloids commonly found in lupins, such as lupanine and sparteine
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in '' Lupinus mutabilis'', and is thought to chelate the bivalent cations calcium and ...
, shows a lower toxicity. Lupinine, with a minimal lethal dose
In toxicology, the lethal dose (LD) is an indication of the lethal toxicity of a given substance or type of radiation. Because resistance varies from one individual to another, the "lethal dose" represents a dose (usually recorded as dose per kilog ...
of 28–30 mg/kg and a toxic dose of 25–28 mg/kg, is about 85 percent as toxic as d-lupanine and about 90% as toxic as sparteine. The relative toxicity of lupinine with other quinolizidine alkaloids commonly found in lupins is shown in the table below.
Mechanism of action
 Studies on the hydrochloride of lupinine have shown it to be a reversible inhibitor of acetylcholinesterases. Lupinine, a nitrogen-containing
Studies on the hydrochloride of lupinine have shown it to be a reversible inhibitor of acetylcholinesterases. Lupinine, a nitrogen-containing heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
, has a structure similar to the ammonium "head" of the acetylcholinesterase endogenous agonist, acetylcholine. At physiological pH, the amine of lupinine is protonated which leads to ion-ion interaction with the acetylcholinesterase anionic site in the same manner as the ammonium on acetylcholine interacts. Previous studies of reversible ammonium inhibitors similar to lupinine have shown that the ammonium groups (corresponding to the protonated amine of lupinine) enter the gorge of the active center of the acetylcholinesterase in the region of the Trp84 residue. This leads to the formation of an enzyme-sorption complex with the anionic portion of the acetylcholinesterase located on the active site of lupinine, namely the amine. This complex blocks the access of acetylcholine to the active center which decreases the catalytic hydrolysis and subsequent breakdown of acetylcholine by acetylcholinesterase. Enzyme inactivation leads to an accumulation of acetylcholine in the body, hyperstimulation of both the muscarinic
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-rec ...
and nicotinic
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral ner ...
acetylcholine receptors, as well as subsequent disruption of neurotransmission
Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), ...
. However, it was found that the time of incubation did not affect the inhibition, leading to the conclusion that lupinine is a reversible inhibitor.
Studies have also shown that lupinine has a binding affinity for both muscarinic
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-rec ...
and nicotinic
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral ner ...
acetylcholine receptors. Lupinine was found to have an IC50 value of >500 μM for nicotinic receptors and an IC50 value of 190 μM for muscarinic receptors. However, it has yet to be determined whether this affinity is agonistic or antagonistic in nature.
Synthesis

Biological
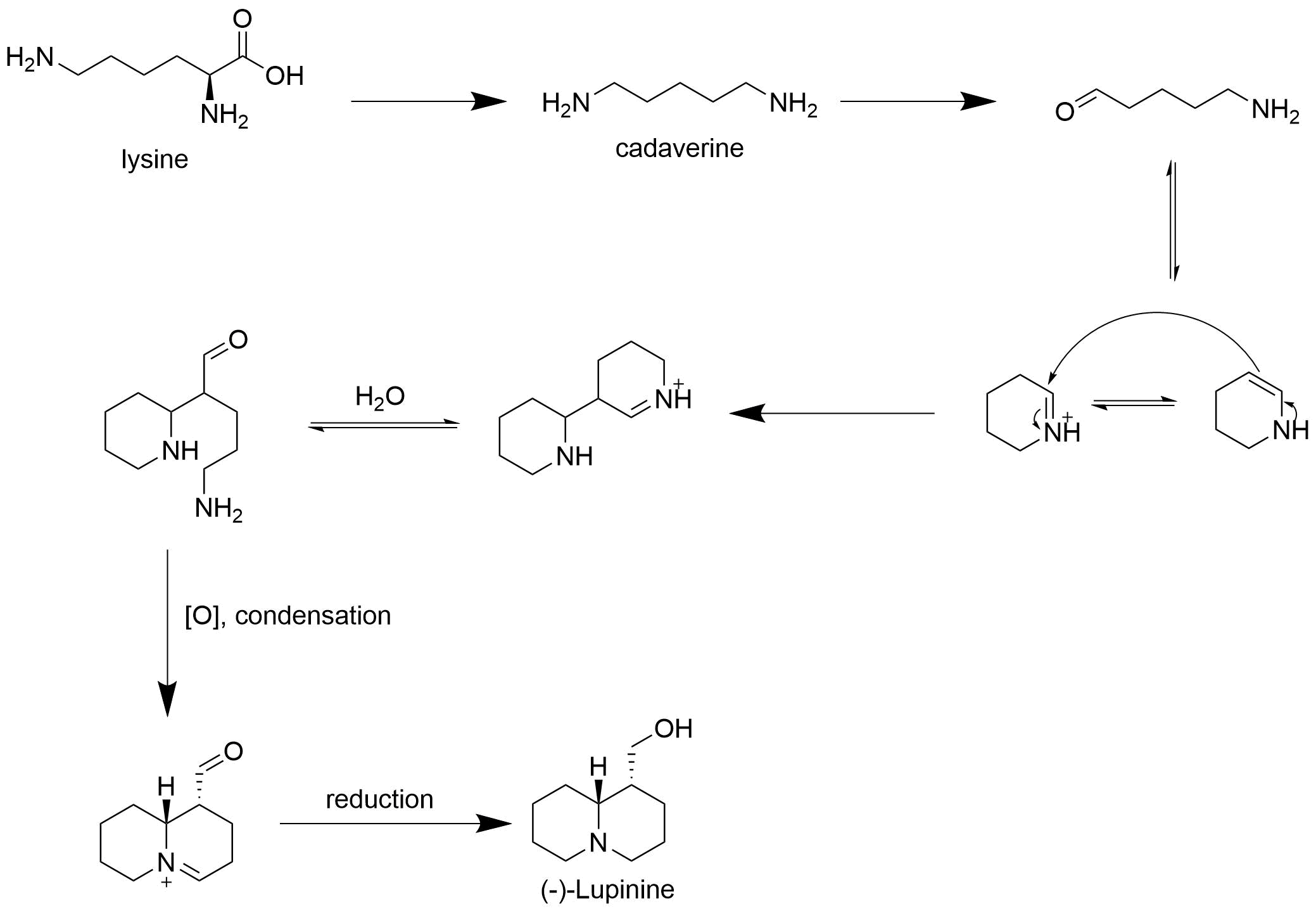
Lupinine is naturally biosynthesized from l-lysine in the ''Lupinus'' genes of plants along with various otherquinolizidine
Quinolizidine (norlupinane, octahydro-2''H''-quinolizine) is a nitrogen-containing heterocyclic compound. Some alkaloids (e.g. cytisine and sparteine) are derivatives of quinolizidine.
Quinolizidine alkaloids
Quinolizidine alkaloids, such as ...
alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s. In the biosynthetic process, lysine is first decarboxylated into cadaverine, which is then oxidatively deaminated to the corresponding aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
. The aldehyde is then spontaneously cyclized into two tautmers which couple through an aldol
In organic chemistry, an aldol describes a structural motif consisting of a 3-hydroxy ketone or 3-hydroxyaldehyde. Aldols are usually the product of aldol addition. When used alone, the term "aldol" may refer to 3-hydroxybutanal.
Stereochemistry
...
type mechanism in which the allylic amine attacks the iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
, forming a dissymmetric dimeric intermediate which is then hydrated. The primary amine is then oxidized and an intramolecular condensation occurs, giving the quinolizidinealdehyde. The aldehyde is then reduced to an alcohol, giving, enantioselectively, (-)- lupinine.
Synthetic
Lupinine has achiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
carbon atom; therefore, total syntheses of lupinine need to be enantioselective for (-)-lupinine in order to provide the biologically active product. The first racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
total synthesis of lupinine occurred in 1937 by Clemo, Morgan, and Raper. Six more total syntheses of lupinine followed between 1940-1956, with the first enantioselective synthesis of lupinine occurring in 1966 by Goldberg and Ragade. Since that initial enantioselective synthesis, there have been numerous total syntheses of both enantio-pure and racemic lupinine. One synthesis, notable because it describes the preparation of all four stereoisomers
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms ...
of lupinine, and containing many references to earlier work in this field, was published by Ma and Ni. Another total synthesis of specific note due to the enantioselectivity and limited number of steps is by Santos et al. In 2010, Santos et al. synthesized enantioselective (-)- lupinine in 36% yield over eight steps using a double Mitsunobu Reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylat ...
. First, they employed asymmetric addition of the starting materials using a Lewis acid, followed by treatment with a reducing agent and a base. This gave the (R,R)-alcohol. This configuration was inverted using a Mitsunobu reaction followed by hydrolysis, affording the (R,S) configuration of the alcohol. The alcohol was then reduced with alane, underwent another Mitsunobu reaction, was hydrolyzed to the acid and finally reduced to (-)-lupinine via alane reduction.

Isolation
One of the earliest isolations of lupinine, from ''Lupinus palmeri'' collected in Utah, USA, is that reported by Couch, who was able to obtain crystalline lupinine without the use ofchromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
techniques.
Applications

Pest control
Lupinine is an insect antifeedant. Studies of its insecticide activity have shown it to be effective against culicine mosquito larvae which are vectors for viruses, filarial worms, and avianmalaria
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. S ...
.
Botany
Lupins are often found growing withCastilleja
''Castilleja'', commonly known as paintbrush, Indian paintbrush, or prairie-fire, is a genus of about 200 species of annual and perennial herbaceous plants native to the west of the Americas from Alaska south to the Andes, northern Asia, and on ...
(Indian paintbrush) which uses lupins as a host and confers lupinine and other alkaloids to itself. This works in tandem with the increase in nitrogen fixation to increase parasitic reproduction rates and potentially reduce herbivory activity; however, studies have shown mixed results in the efficacy of alkaloid transfer in prevention of herbivory activity.
Regulations
TheEuropean Chemicals Agency
The European Chemicals Agency (ECHA; ) is an agency of the European Union which manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restrict ...
(ECA) labels lupinine under the hazard statement codes H302, H312, and H332, which indicate that lupinine is harmful if swallowed, harmful in contact with skin, and harmful if inhaled, respectively. It is given a GHS07 labeling which indicates its acute oral toxicity is category 4.
See also
* Lupin poisoning *Sparteine
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in '' Lupinus mutabilis'', and is thought to chelate the bivalent cations calcium and ...
References
External links
*{{Commons category-inline Quinolizidine alkaloids Primary alcohols Alkaloids found in Fabaceae Plant toxins