|
Low Density Polyethylene
Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by John C. Swallow and M.W Perrin who were working for Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its manufacture employs the same method today. The EPA estimates 5.7% of LDPE ( resin identification code 4) is recycled in the United States. Despite competition from more modern polymers, LDPE continues to be an important plastic grade. In 2013 the worldwide LDPE market reached a volume of about US$33 billion. Despite its designation with the recycling symbol, it cannot be as commonly recycled as No. 1 (polyethylene terephthalate) or 2 plastics (high-density polyethylene). Properties LDPE is defined by a density range of 917–930 kg/m3. At room temperature it is not reactive, except to strong oxidizers; some solvents cause it to swell. It can withstand temperatures of continuous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol Resin Code 4 LDPE
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise different concepts and experiences. All communication is achieved through the use of symbols: for example, a red octagon is a common symbol for " STOP"; on maps, blue lines often represent rivers; and a red rose often symbolizes love and compassion. Numerals are symbols for numbers; letters of an alphabet may be symbols for certain phonemes; and personal names are symbols representing individuals. The academic study of symbols is called semiotics. In the arts, symbolism is the use of a concrete element to represent a more abstract idea. In cartography, an organized collection of symbols forms a legend for a map. Etymology The word ''symbol'' derives from the late Middle French masculine noun , which appeared around 1380 in a theological sense signifying a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-density Polyethylene
High-density polyethylene (HDPE) or polyethylene high-density (PEHD) is a thermoplastic polymer produced from the monomer ethylene. It is sometimes called "alkathene" or " polythene" when used for HDPE pipes. With a high strength-to-density ratio, HDPE is used in the production of plastic bottles, corrosion-resistant piping, geomembranes and plastic lumber. HDPE is commonly recycled, and has the number "2" as its resin identification code. In 2008, the global HDPE market reached a volume of more than 30 million tons. Properties HDPE is known for its high strength-to-density ratio. The density of HDPE ranges from 930 to 970 kg/m3. Although the density of HDPE is only marginally higher than that of low-density polyethylene, HDPE has little branching, giving it stronger intermolecular forces and tensile strength (38 MPa versus 21 MPa) than LDPE. The difference in strength exceeds the difference in density, giving HDPE a higher specific strength. It is also harder and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and '' Lewis bases''. All definitions agree that bases are substances that react with acid An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...s, as originally proposed by Guillaume-François Rouelle, G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−. These ions can react with Hydron (chemistry), hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an Organic chemistry, organic Organic compound, compound, and among the simplest of organic compounds. Methane is also a hydrocarbon. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the Atmosphere of Earth, atmosphere, it is known as atmospheric methane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about , rather than the present average of .Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007:Chapter 1: Historical Overview of Climate Change. In:Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. olomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller (eds.) Cambridge University Press, Cambridge, United Kingdom and New Y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
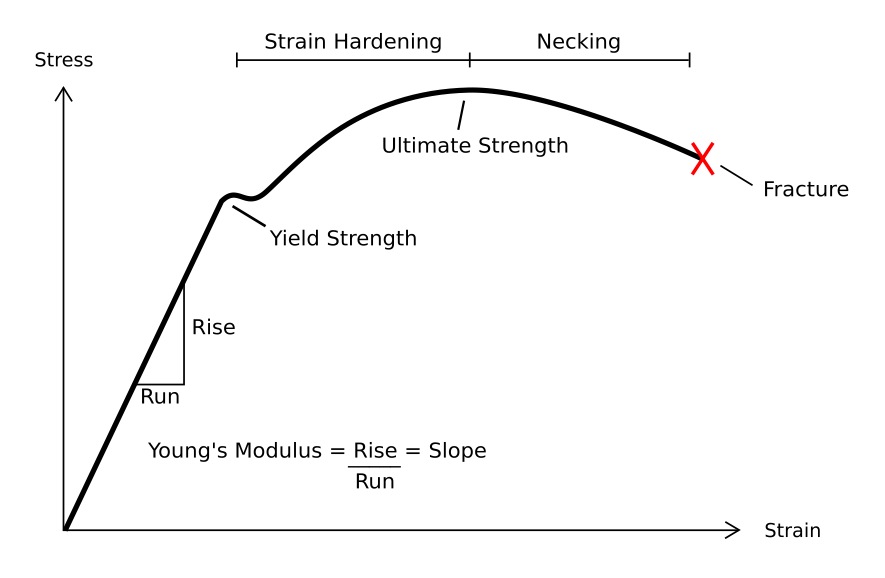
Resilience (materials Science)
In material science, resilience is the ability of a material to absorb energy when it is deformed elastically, and release that energy upon unloading. Proof resilience is defined as the maximum energy that can be absorbed up to the elastic limit, without creating a permanent distortion. The modulus of resilience is defined as the maximum energy that can be absorbed per unit volume without creating a permanent distortion. It can be calculated by integrating the stress–strain curve from zero to the elastic limit. In uniaxial tension, under the assumptions of linear elasticity, : U_= \frac = \frac where ''Ur'' is the modulus of resilience, ''σy'' is the yield strength, ''εy'' is the yield strain, and ''E'' is the Young's modulus. This analysis is not valid for non-linear elastic materials like rubber, for which the approach of area under the curve until elastic limit must be used. Unit of resilience Modulus of resilience (''U''r) is measured in a unit of joule per cubic m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |