|
List Of Vaccine Topics
This is a list of vaccine-related topics. A vaccine is a biological preparation that improves immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism, and is often made from weakened or killed forms of the microbe or its toxins. The agent stimulates the body's immune system to recognize the agent as foreign, destroy it, and "remember" it, so that the immune system can more easily recognize and destroy any of these microorganisms that it later encounters. Human vaccines Viral diseases Bacterial diseases Vaccines under research Viral diseases * Adenovirus vaccine * COVID-19 vaccine ( Part of today's pandemic since 2019) * Coxsackie B virus vaccine * Cytomegalovirus vaccine * Chikungunya vaccine * Eastern Equine encephalitis virus vaccine for humans * Enterovirus 71 vaccine * Epstein–Barr vaccine * H5N1 vaccine * Hepatitis C vaccine * HIV vaccine * HTLV-1 T-lymphotropic leukemia vaccine for humans * Marbur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
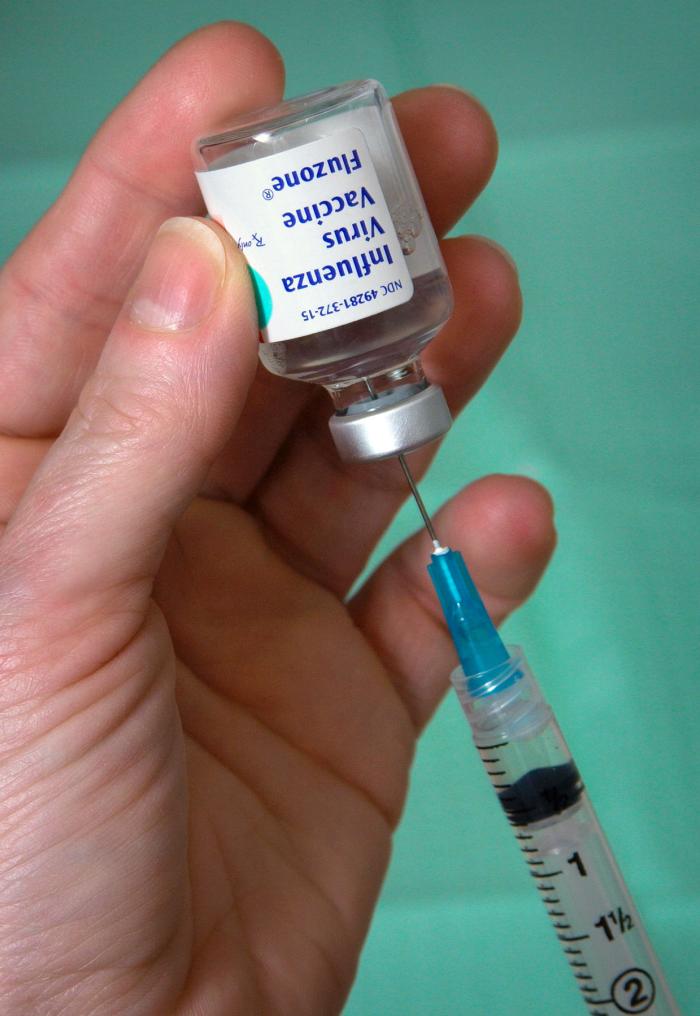
Fluzone Vaccine Extracting
Influenza vaccines, also known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research. Vaccinating children may protect those around them. Vaccines are an effective means to control outbreaks of many diseases. However, vaccines for respiratory viral infections such as flu are still suboptimal and do not offer broad-spectrum protection. Vaccination against influenza began in the 1930s, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ZF2001
ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan. ZF2001 employs technology similar to other protein-based vaccines in Phase III trials from Novavax, Vector Institute, and Medicago. ZF2001 was first approved for use in Uzbekistan and later China. Production capacity is expected to be one billion doses a year in China and 200 million in Uzbekistan. By July, 100 million doses had been administered in China and Uzbekistan. Medical uses It is administered in three doses over a period of two months. Efficacy In August 2021, preliminary data from a phase III study with 28,500 participants indicated an overall efficacy of 82% against disease of any severity. Efficacy was 93% against the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2009 Swine Flu Pandemic Vaccine
The 2009 swine flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed virus was injected, while the live virus was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009. In studies, the vaccine appeared both effective and safe, providing a strong protective immune response and having a similar safety profile to the usual seasonal influenza vaccine. However, about 30% of people already had some immunity to the virus, with the vaccine conferring greatest benefit on young people, since many older people are already immune through exposure to similar viruses in the past. The vaccine also provided some cross-protection against the 1918 flu pandemic strain. Early results (pre-25 De ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RVSV-ZEBOV Vaccine
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain. rVSV-ZEBOV is a recombinant, replication-competent viral vector vaccine. It consists of rice-derived recombinant human serum albumin and live attenuated recombinant vesicular stomatitis virus (VSV), which has been genetically engineered to express the main glycoprotein from the Zaire ebolavirus so as to provoke a neutralizing immune response to the Ebola virus. The vaccine was approved for medical use in the European Union and the United States in 2019. It was created by scientists at the National Microbiology Laboratory in Winnipeg, Manito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
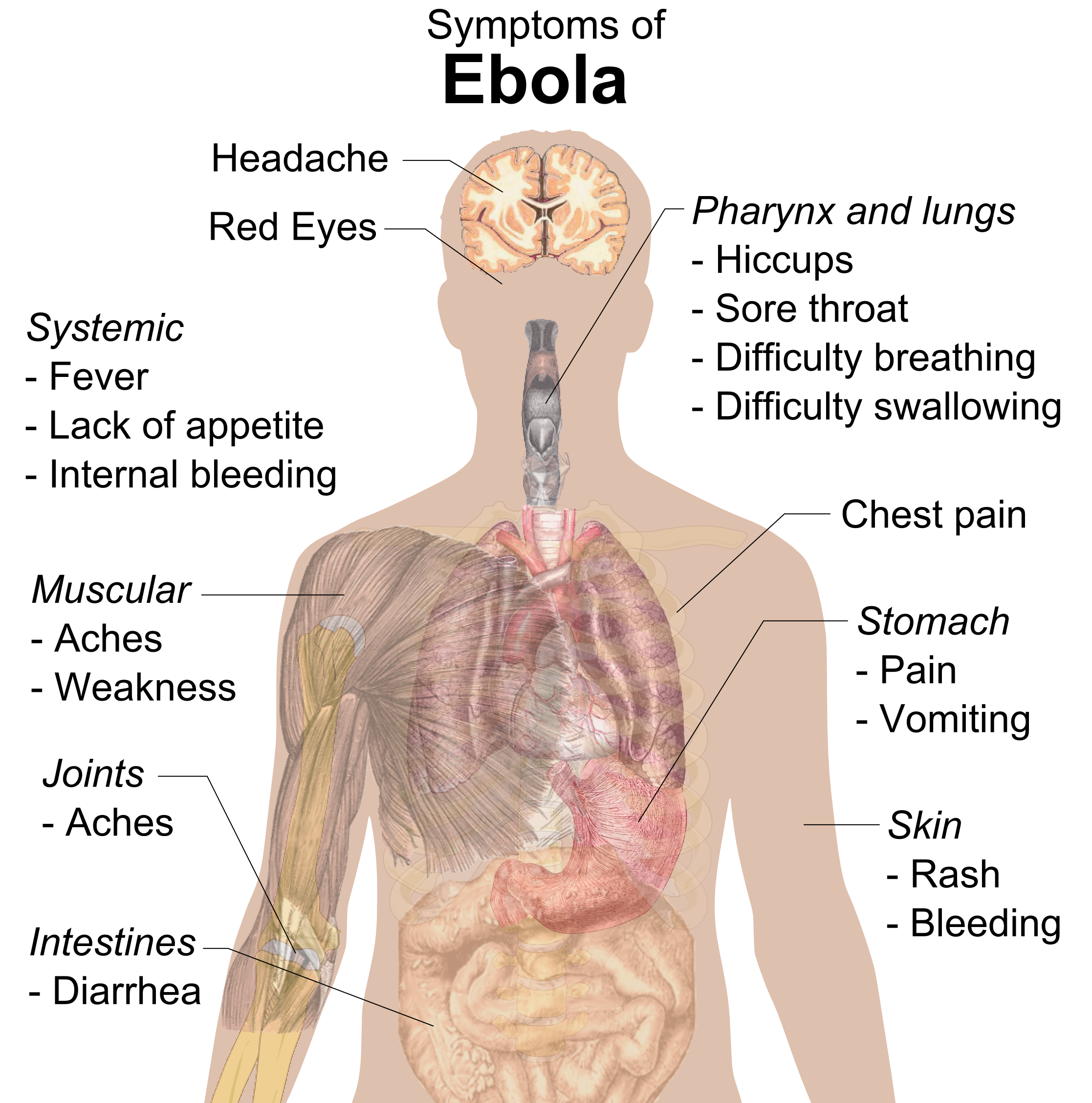
Ebola
Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses. Symptoms typically start anywhere between two days and three weeks after becoming infected with the virus. The first symptoms are usually fever, sore throat, muscle pain, and headaches. These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function, at which point, some people begin to bleed both internally and externally. The disease kills between 25% and 90% of those infected – about 50% on average. Death is often due to shock from fluid loss, and typically occurs between six and 16 days after the first symptoms appear. Early treatment of symptoms increases the survival rate considerably compared to late start. The virus spreads through direct contact with body fluids, such as blood from infected humans or other animals, or from contact with items that have recently been conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ebolavirus
The genus ''Ebolavirus'' (- or ; - or ) is a virological taxon included in the family ''Filoviridae'' (filament-shaped viruses), order ''Mononegavirales''. The members of this genus are called ebolaviruses, and encode their genome in the form of single-stranded negative-sense RNA. The six known virus species are named for the region where each was originally identified: ''Bundibugyo ebolavirus'', '' Reston ebolavirus'', '' Sudan ebolavirus'', ''Taï Forest ebolavirus'' (originally ''Côte d'Ivoire ebolavirus''), ''Zaire ebolavirus'', and ''Bombali ebolavirus''. The last is the most recent species to be named and was isolated from Angolan free-tailed bats in Sierra Leone. Each species of the genus ''Ebolavirus'' has one member virus, and four of these cause Ebola virus disease (EVD) in humans, a type of hemorrhagic fever having a very high case fatality rate. The Reston virus has caused EVD in other primates. ''Zaire ebolavirus'' has the highest mortality rate of the ebolaviru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ebola Vaccine
Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates (usually macaques) against lethal infection. Vaccines include replication-deficient adenovirus vectors, replication-competent vesicular stomatitis (VSV) and human parainfluenza (HPIV-3) vectors, and virus-like nanoparticle preparations. Conventional trials to study efficacy by exposure of humans to the pathogen after immunization are not ethical in this case. For such situations, the US Food and Drug Administration (FDA) has established the "animal efficacy rule" allowing licensure to be approved on the basis of animal model studies that replicate huma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dengue Fever
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. Symptoms typically begin three to fourteen days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin itching and skin rash. Recovery generally takes two to seven days. In a small proportion of cases, the disease develops into a more severe dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and blood plasma leakage, or into dengue shock syndrome, where dangerously low blood pressure occurs. Dengue is spread by several species of female mosquitoes of the ''Aedes'' genus, principally ''Aedes aegypti''. The virus has five serotypes; infection with one type usually gives lifelong immunity to that type, but only short-term immunity to the others. Subsequent infection with a different type increases the risk of severe complications. A number of tests are available to confirm the diagnosis including detecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
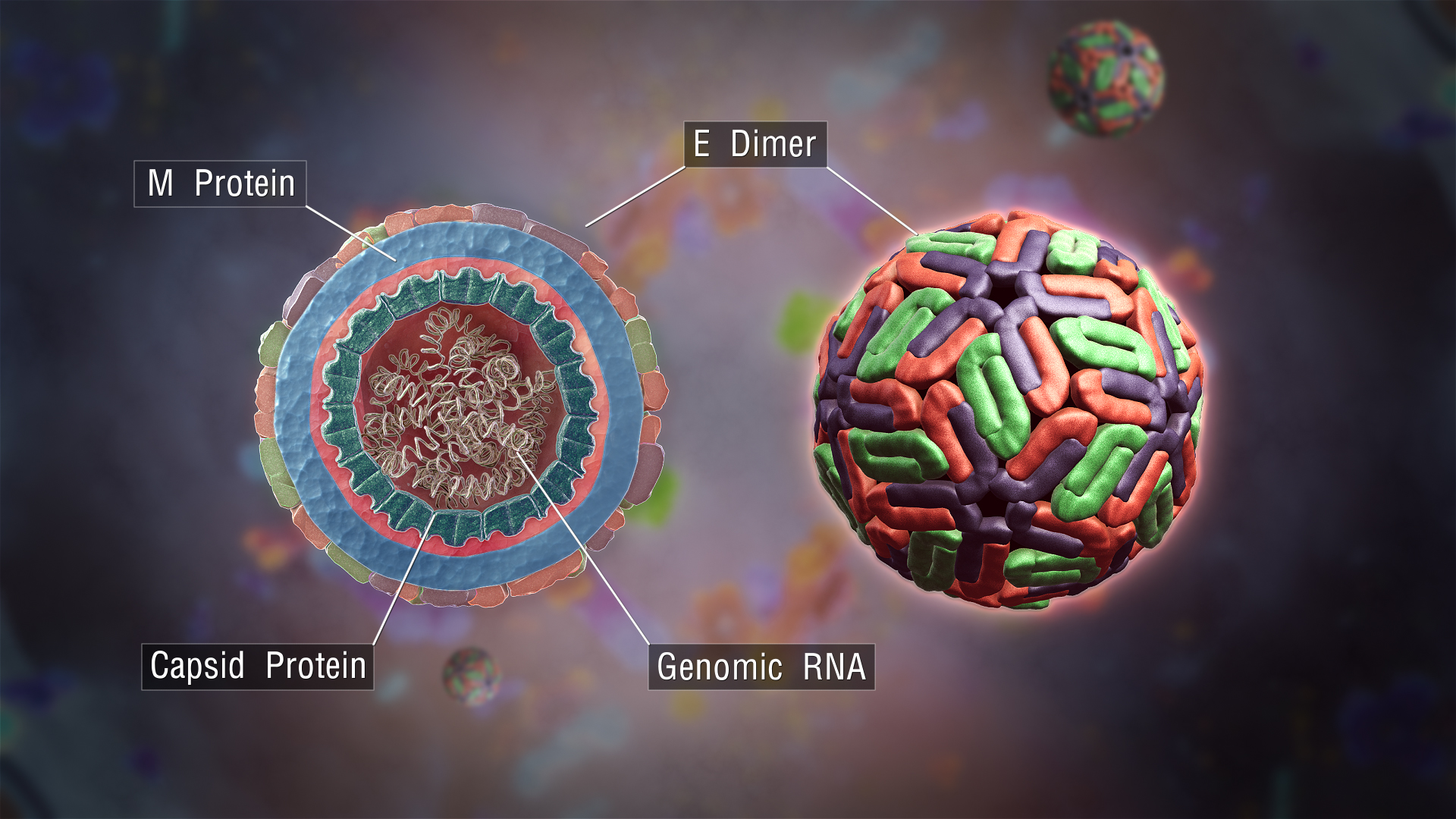
Dengue Virus
''Dengue virus'' (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family ''Flaviviridae''; genus ''Flavivirus''. Four serotypes of the virus have been found, a reported fifth has yet to be confirmed,Dwivedi, V. D., Tripathi, I. P., Tripathi, R. C., Bharadwaj, S., & Mishra, S. K. (2017). Genomics, proteomics and evolution of ''Dengue virus''. Briefings in functional genomics.16(4): 217–227, https://doi.org/10.1093/bfgp/elw040 all of which can cause the full spectrum of disease. Nevertheless, scientists' understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a ''continuum'' appears to exist. This same study identified 47 strains of ''dengue virus''. Additionally, coinfection with and lack of rapid tests for ''zika virus'' and ''chikungunya'' complicate matters in real-world infections. ''Dengue virus'' has increased dramatically within the last 20 years, becoming one of the worst mosquito- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dengue Vaccine
Dengue vaccine is a vaccine used to prevent dengue fever in humans. Development of dengue vaccines began in the 1920s, but was hindered by the need to create immunity against all four dengue serotypes. As of 2022, there are two commercially available vaccine and sold under the brand name Dengvaxia and Qdenga. Dengvaxia is only recommended in those who have previously had dengue fever or populations in which most people have been previously infected. The value of Dengavaxia is limited by the fact that it may increase the risk of severe dengue in those who have not previously been infected. In 2017, more than 733,000 children and more than 50,000 adult volunteers were vaccinated with CYD-TDV regardless of serostatus, which led to a controversy. Qdenga is designated for people not previously infected. There are other vaccine candidates in development including live attenuated, inactivated, DNA and subunit vaccines. History In December 2018, Dengvaxia was approved in the Europe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxford–AstraZeneca COVID-19 Vaccine
The Oxford–AstraZeneca COVID19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose, and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant (lineage B.1.1.7), and 61% against the Delta variant (lineage B.1.617.2). The vaccine is stable at refrigerator temperatures and has a good safety profile, with side effects including injection-site pain, headache, and nausea, all generally resolving within a few days. More rarely, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sputnik V COVID-19 Vaccine
Sputnik V (russian: Спутник V, the brand name from RDIF) or Gam-COVID-Vac (russian: Гам-КОВИД-Вак, the name under which it is legally registered and produced) is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is the world's first registered combination vector vaccine for the prevention of COVID-19, having been registered on 11 August 2020 by the Russian Ministry of Health. Gam-COVID-Vac was initially approved for distribution in Russia and then in 59 other countries (as of April 2021) on the preliminary results of Phase I– II studies eventually published on 4 September 2020. Approval in early August of Gam-COVID-Vac was met with criticism in mass media and discussions in the scientific community as to whether approval was justified in the absence of robust scientific research confirming safety and efficacy. A large-scale Brazilian study from Dec. 2020 to May 2021 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_2021_C.jpg)
.jpg)