|
List Of HU Cannabinoids
A research group led by Raphael Mechoulam at Hebrew University has synthesized many cannabinoids. Some of those are: * HU-210 — a high affinity CB1 agonist (''K''i = 0.23 nM) * HU-211 — the (+)-enantiomer of HU-210 with dramatically reduced CB1 affinity * HU-239 — also known as ajulemic acid * HU-243 * HU-308 * HU-320 * HU-331 * HU-336 * HU-345 See also * List of AM cannabinoids * List of CP cannabinoids * List of JWH cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{DEFAULTSORT:HU cannabinoids HU Cannabis-related lists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raphael Mechoulam
Raphael Mechoulam ( he, רפאל משולם, bg, Рафаел Мешулам; born 5 November 1930) is an Israeli organic chemist and professor of Medicinal Chemistry at the Hebrew University of Jerusalem in Israel. Mechoulam is best known for his work (together with Y. Gaoni) in the isolation, structure elucidation and total synthesis of Δ9-tetrahydrocannabinol, the main active principle of cannabis and for the isolation and the identification of the endogenous cannabinoids anandamide from the brain and 2-arachidonoyl glycerol (2-AG) from peripheral organs together with his students, postdocs and collaborators. Biography Mechoulam was born in Sofia, Bulgaria in 1930, to a Sephardic Jewish family. His father was a physician and head of a local hospital, while his mother "who had studied in Berlin, enjoyed the life of a well-to-do Jewish family". He attended an "American grade school" until his parents were forced to leave their hometown because of anti-semitic laws and his fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-331
HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the ''Cannabis sativa'' plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti-neoplastic activity while maintaining low toxicity at therapeutic doses. Mechanism of action Inhibitors of topoisomerases can act at two different levels. First inhibiting topoisomerase, which stabilize the topoisomerase-DNA complex and thus introduce DNA breaks in the wires that lead to apoptosis, then inhibiting the catalytic activity of topo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
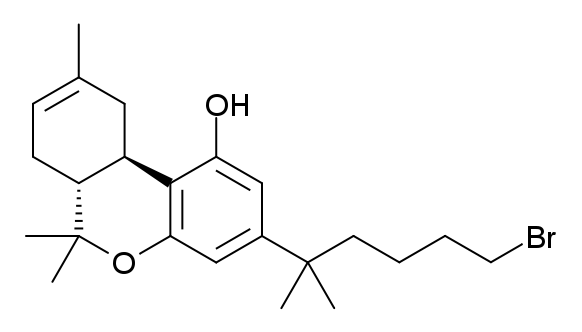
List Of Miscellaneous Designer Cannabinoids
Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally designed by clandestine medicinal chemists. These unprecedented synthetic cannabinoids often feature alphanumeric code names intended to mimic the style of chemical nomenclature used by academic laboratories and pharmaceutical companies, and there is generally little, if any, information available regarding their pharmacology and toxicology at the time of first discovery. * 5F-AB-PINACA — the terminally fluorinated (5-fluoropentyl) analogue of AB-PINACA. * 5F-AMB — * 5F-ADB — * 5F-NNE1 — * 5F-PCN — * 5F-SDB-006 — the terminally fluorinated (5-fluoropentyl) analogue of SDB-006. * AB-001 — one of the earliest adamantane derivatives discovered as a designer cannabinoid. AB-001 was unknown in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of JWH Cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
List Of CP Cannabinoids
Many synthetic cannabinoids were designed by Pfizer in the 1970s and 1980s, and feature an alphanumeric code beginning with the prefix "CP" (after Charles Pfizer). Recently, several members of this class of cannabinoids have been discovered in recreational drug products. * CP 47,497 — * (C6)-CP 47,497 — * (C7)-CP 47,497 (CP 47,497 itself) — * (C8)-CP 47,497 (Cannabicyclohexanol) — * (C9)-CP 47,497 — * CP 50,556-1 (Levonantradol) — * CP 55,244 — * CP 55,940 — * (±)-CP 55,940 — (±)-CP 55,940 is a widely used cannabinoid research tool. * (+)-CP 55,940 — * (-)-CP 55,940 — * CP-945,598 (Otenabant) — See also * List of AM cannabinoids * List of HU cannabinoids * List of JWH cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature contin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-345
HU-345 (cannabinol quinone) is a drug that is able to inhibit aortic ring angiogenesis more potently than its parent compound cannabinol (CBN). It exhibits no psychoactive effects on the body. HU-345 can be derived through the oxidative degradation of CBN. See also *HU-331 HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the ''Cannabis sativa'' plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspas ... * HU-336 References {{cannabinoid-stub Angiogenesis inhibitors HU cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-336
HU-336 is a strongly antiangiogenic compound, significantly inhibiting angiogenesis at concentrations as low as 300 nM. It inhibits angiogenesis by directly inducing apoptosis of vascular endothelial cells without changing the expression of pro- and antiangiogenic cytokines and their receptors. HU-336 is highly effective against tumor xenografts in nude mice. Although it is technically the oxidized quinone of delta-8 THC, it is entirely non psychoactive. See also *HU-331 *HU-345 HU-345 (cannabinol quinone) is a drug that is able to inhibit aortic ring angiogenesis more potently than its parent compound cannabinol (CBN). It exhibits no psychoactive effects on the body. HU-345 can be derived through the oxidative degr ... References {{cannabinoid-stub Angiogenesis inhibitors HU cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-320
HU-320 (7-nor-7-carboxy-CBD-1,1-DMH) is a drug related to cannabidiol, which has strong antiinflammatory and immunosuppressive properties while demonstrating no psychoactive effects. See also * 7-Hydroxycannabidiol * Ajulemic acid * HU-210 * HU-308 * HU-331 HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the ''Cannabis sativa'' plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspas ... References HU cannabinoids Cyclohexenes 2,6-Dihydroxybiphenyls {{pharmacology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hebrew University
The Hebrew University of Jerusalem (HUJI; he, הַאוּנִיבֶרְסִיטָה הַעִבְרִית בִּירוּשָׁלַיִם) is a public research university based in Jerusalem, Israel. Co-founded by Albert Einstein and Dr. Chaim Weizmann in July 1918, the public university officially opened in April 1925. It is the second-oldest Israeli university, having been founded 30 years before the establishment of the State of Israel but six years after the older Technion university. The HUJI has three campuses in Jerusalem and one in Rehovot. The world's largest library for Jewish studies—the National Library of Israel—is located on its Edmond J. Safra campus in the Givat Ram neighbourhood of Jerusalem. The university has five affiliated teaching hospitals (including the Hadassah Medical Center), seven faculties, more than 100 research centers, and 315 academic departments. , one-third of all the doctoral candidates in Israel were studying at the HUJI. Among its first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-308
HU-308 (also known as onternabez, HU308, PPP-003 and ARDS-003) is a cannabidiol (CBD)-derivative drug that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor. The synthesis and characterization of HU-308 took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative HU-308 was identified as a potent peripheral CB2-selective agonist in ''in vitro'' and animal studies in 1990 and 1999. Legal status Tetra Bio-Pharma owns the intellectual property rights to HU-308. HU-308 is non-psychoactive and not scheduled at the federal level in the United States. It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there. References {{Cannabinoids See also * HU-210 * HU-320 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-243
HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay. Legal status HU-243 is not listed in the schedules set out by the United Nations' Single Convention on Narcotic Drugs from 1961 nor their Convention on Psychotropic Substances from 1971, so the signatory countries to these international drug control treaties are not required by said treaties to control HU-243. United States HU-243 is not listed in the list of scheduled controlled substances in the USA. It is therefore not scheduled at the federal level in the United States, but it is possible that HU-243 could legally be considered an analog of THC (which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |