|
List Of Alloys
This is a list of named alloys grouped alphabetically by base metal. Within these headings, the alloys are also grouped alphabetically. Some of the main alloying elements are optionally listed after the alloy names. Alloys by base metal Aluminium * AA-8000: used for electrical building wire in the U.S. per the National Electrical Code, replacing AA-1350. * Al–Li (2.45% lithium): aerospace applications, including the Space Shuttle * Alnico (nickel, cobalt): used for permanent magnets * Aluminium–Scandium (scandium) * Birmabright (magnesium, manganese): used in car bodies, mainly used by Land Rover cars. * Duralumin (copper) * Hiduminium or R.R. alloys (2% copper, iron, nickel): used in aircraft pistons * Hydronalium (up to 12% magnesium, 1% manganese): used in shipbuilding, resists seawater corrosion * Italma (3.5% magnesium, 0.3% manganese): formerly used to make coinage of the Italian lira * Magnalium (5-50% magnesium): used in airplane bodies, ladders, pyrotechnics, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Alloys are defined by a metallic bonding character. The alloy constituents are usually measured by mass percentage for practical applications, and in atomic fraction for basic science studies. Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy. They can be further classified as homo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hiduminium
The Hiduminium alloys or R.R. alloys are a series of high-strength, high-temperature aluminium alloys, developed for aircraft use by Rolls-Royce ("RR") before World War II. They were manufactured and later developed by High Duty Alloys Ltd. The name ''Hi''-''Du''-Minium is derived from that of ''Hi''gh ''Du''ty Alu''minium'' Alloys. The first of these Hiduminium alloys was termed '' 'R.R.50' ''. This alloy was first developed for motor-racing pistons, and was only later adopted for aircraft engine use. It was a development of the earlier Y alloy, the first of the nickel-containing light aluminium alloys. These alloys are one of the three main groups of high-strength aluminium alloys, the nickel-aluminium alloys having the advantage of retaining strength at high temperatures, making them particularly useful for pistons. Early adoption The alloys were in limited use for aircraft by 1929, being used in the Rolls-Royce R engine that was successful in the Schneider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerrosafe
Cerrosafe is a fusible alloy with a low melting point. It is a non- eutectic mixture consisting of 42.5% bismuth, 37.7% lead, 11.3% tin, and 8.5% cadmium that melts between and . It is useful for making reference castings whose dimensions can be correlated to those of the mold or other template due to its well-known thermal expansion Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions. Temperature is a monotonic function of the average molecular kinetic ... properties during cooling. The alloy contracts during the first 30 minutes, allowing easy removal from a mold, then expands during the next 30 minutes to return to the exact original size. It then continues expanding at a known rate for 200 hours, allowing conversion of measurements of the casting back to those of the mold. Similar metals References External links * Examples ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismanol
Bismanol is an magnetic alloy of bismuth and manganese (manganese bismuthide) developed by the US Naval Ordnance Laboratory. History Bismanol, a permanent magnet made from powder metallurgy of manganese bismuthide, was developed by the US Naval Ordnance Laboratory in the early 1950s – at the time of invention it was one of the highest coercive force permanent magnets available, at 3000 oersteds. Coercive force reached 3650 oersteds and magnetic flux density 4800 by the mid 1950s. The material was generally strong, and stable to shock and vibration, but had a tendency to chip. Slow corrosion of the material occurred under normal conditions. The material was used to make permanent magnets for use in small electric motors. Bismanol magnets have been replaced by neodymium magnets which are both cheaper and superior in other ways, by samarium-cobalt magnets in more critical applications, and by alnico Alnico is a family of iron alloys which in addition to iron are compose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lockalloy
Lockalloy is an alloy that consists of 62% beryllium and 38% aluminum. It was used as a structural metal in the aerospace industry because of its high specific strength and stiffness. The alloy was first developed in the 1960s by the Lockheed Missiles and Space Company. The material was used in the Lockheed YF12 and LGM-30 Minuteman The LGM-30 Minuteman is an American land-based intercontinental ballistic missile (ICBM) in service with the Air Force Global Strike Command. , the LGM-30G Minuteman III version is the only land-based ICBM in service in the United States and re ... missile systems. In the 1970s production difficulties limited the material to a few specialized uses and by the mid 1970s Lockalloy was no longer commercially available. In 1990, Materion Beryllium & Composites re-introduced the material into the commercial marketplace as a powder-sintered composite under the trade name of AlBeMet. References {{reflist Beryllium alloys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex Metallic Alloys
Complex metallic alloys (CMAs) or complex intermetallics (CIMs) are intermetallic compounds characterized by the following structural features: #large unit cells, comprising some tens up to thousands of atoms, #the presence of well-defined atom clusters, frequently of icosahedral point group symmetry, #the occurrence of inherent disorder in the ideal structure. Overview ''Complex metallic alloys'' is an umbrella term for intermetallic compounds with a relatively large unit cell. There is no precise definition of how large the unit cell of a complex metallic alloy has to be, but the broadest definition includes Zintl phases, skutterudites, and Heusler compounds on the most simple end, and quasicrystals on the more complex end. Research Following the invention of X-ray crystallography techniques in the 1910s, the atomic structure of many compounds was investigated. Most metals have relatively simple structures. However, in 1923 Linus Pauling reported on the structure of the in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and Passivation (chemistry), forms a protective layer of Aluminium oxide, oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, Magnetism, non-magnetic and ductility, ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of Aluminum-26, 26Al is used in Radiometric dating, radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Y Alloy
Y alloy is a nickel-containing aluminium alloy. It was developed by the British National Physical Laboratory during World War I, in an attempt to find an aluminium alloy that would retain its strength at high temperatures. Duralumin, an aluminium alloy containing 4% copper was already known at this time. Its strength, and its previously unknown age hardening behaviour had made it a popular choice for zeppelins. Aircraft of the period were largely constructed of wood, but there was a need for an aluminium alloy suitable for making engines, particularly pistons, that would have the strength of duralumin but could retain this when in service at high temperatures for long periods. The National Physical Laboratory began a series of experiments to study new aluminium alloys. Experimental series "Y" was successful, and gave its name to the new alloy. Like duralumin, this was a 4% copper alloy, but with the addition of 2% nickel and 1.5% magnesium. This addition of nickel was an innov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
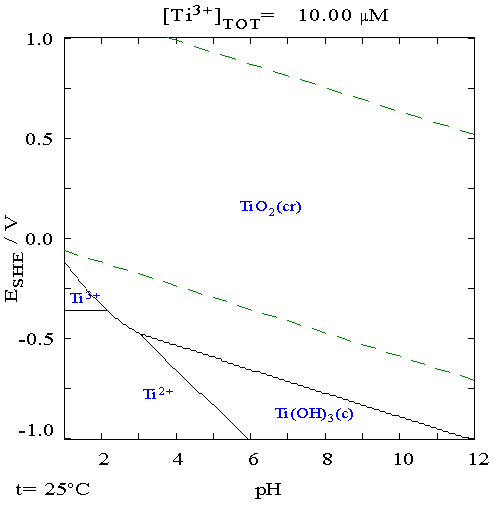
Titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Kingdom of Great Britain, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titan (mythology), Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll process, Kroll and Hunter process, Hunter processes. The most common compound, titanium dioxide, is a popular photocatalysis, photocatalyst and is used in the manufacture of white pigments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnalium
Magnalium is an aluminium alloy with 5% magnesium and 95% aluminum. Properties Alloys with small amounts of magnesium (about 5%) exhibit greater strength, greater corrosion resistance, and lower density than pure aluminium. Such alloys are also more workable and easier to weld than pure aluminium. Alloys with high amounts of magnesium (around 50%) are brittle and more susceptible to corrosion than aluminium. Uses Although they are generally more expensive than aluminium, the high strength, low density, and greater workability of alloys with low amounts of magnesium leads to their use in aircraft and automobile parts. Alloys with about 50% magnesium are brittle and corrode easily, which makes them unsuitable for most engineering uses. However, these alloys are flammable when powdered, are more resistant to corrosion than pure magnesium, and are more reactive than pure aluminium and are therefore used in pyrotechnics as a metal fuel and to produce sparks Sparks may refer to: Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |