|
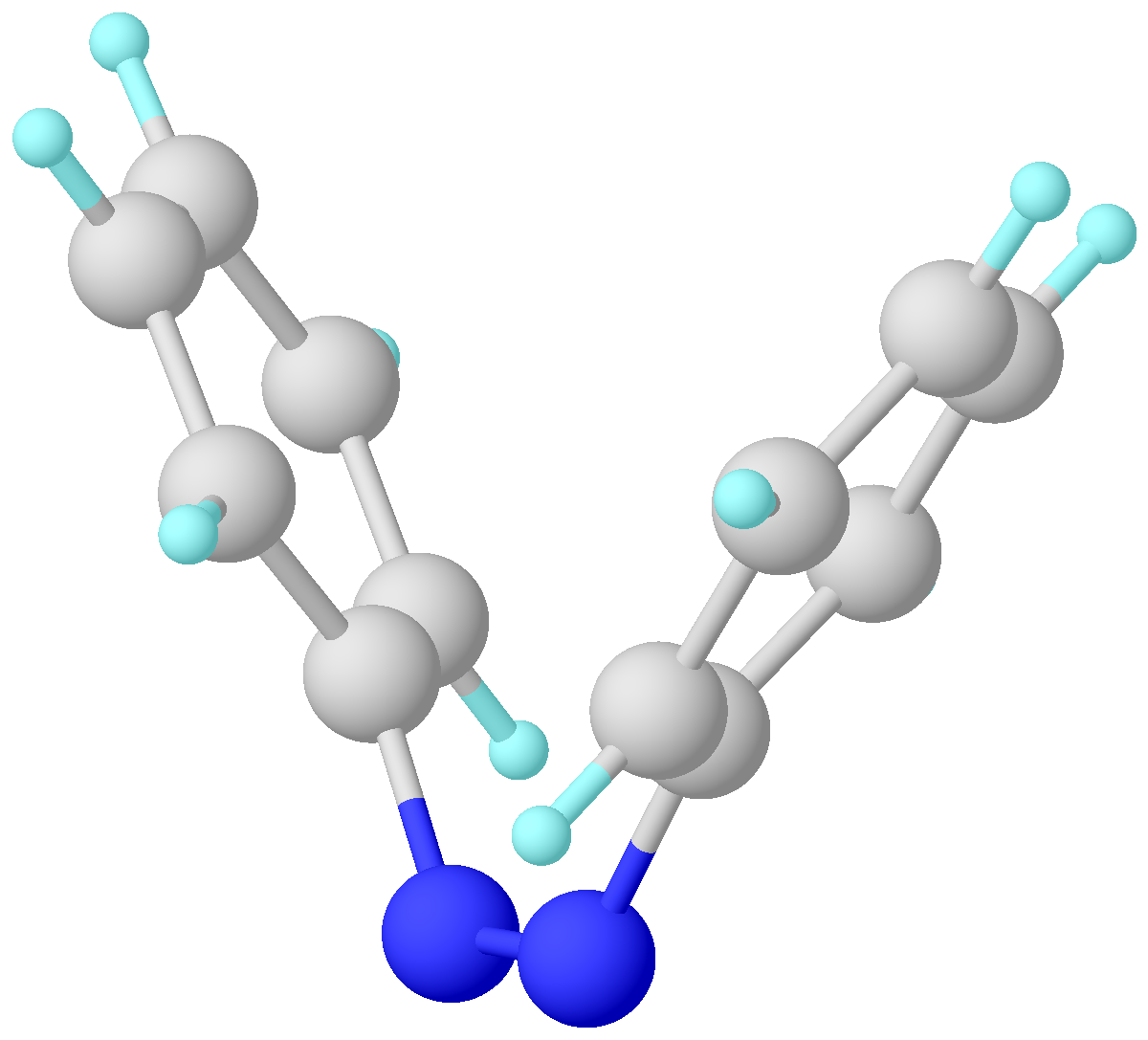
Liquid Crystal Polymer
Liquid crystal polymers (LCPs) are polymers with the property of liquid crystal, usually containing aromatic rings as mesogens. Despite uncrosslinked LCPs, polymeric materials like liquid crystal Elastomer, elastomers (LCEs) and liquid crystal networks (LCNs) can exhibit liquid crystallinity as well. They are both crosslinked LCPs but have different cross link density. They are widely used in the digital display market. In addition, LCPs have unique properties like thermal actuation, anisotropic swelling, and soft elasticity. Therefore, they can be good actuators and sensors. One of the most famous and classical applications for LCPs is Kevlar, a strong but light fiber with wide applications including bulletproof vests. Background Liquid crystallinity in polymers may occur either by dissolving a polymer in a solvent (lyotropic liquid-crystal polymers) or by heating a polymer above its glass or melting transition point (thermotropic liquid-crystal polymers). Liquid-crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermotropic
A liquid crystal phase is thermotropic if its order parameter is determined by temperature. At high temperatures, liquid crystals become an isotropic liquid and at low temperatures, they tend to glassify. In a thermotropic crystal, those phase transitions occur only at temperature extremes; the phase is insensitive to concentration. Most thermotropic liquid crystals are composed of rod-like molecules, and admit nematic, smectic, or cholesterolic phases. References * See also * Thermochromism Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a differen ... * Thermotropic liquid crystals External links What are Liquid Crystals? Liquid crystals {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actuators
An actuator is a component of a machine that is responsible for moving and controlling a mechanism or system, for example by opening a valve. In simple terms, it is a "mover". An actuator requires a control device (controlled by control signal) and a source of energy. The control signal is relatively low energy and may be electric voltage or current, pneumatic, or hydraulic fluid pressure, or even human power. Its main energy source may be an electric current, hydraulic pressure, or pneumatic pressure. The Control device is usually a valve. When it receives a control signal, an actuator responds by converting the source's energy into mechanical motion. In the ''electric'', ''hydraulic'', and ''pneumatic'' sense, it is a form of automation or automatic control. History The history of the pneumatic actuation system and the hydraulic actuation system dates to around the time of World War II (1938). It was first created by Xhiter Anckeleman who used his knowledge of engines and brake ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anisotropy
Anisotropy () is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties (absorbance, refractive index, conductivity, tensile strength, etc.). An example of anisotropy is light coming through a polarizer. Another is wood, which is easier to split along its grain than across it. Fields of interest Computer graphics In the field of computer graphics, an anisotropic surface changes in appearance as it rotates about its geometric normal, as is the case with velvet. Anisotropic filtering (AF) is a method of enhancing the image quality of textures on surfaces that are far away and steeply angled with respect to the point of view. Older techniques, such as bilinear and trilinear filtering, do not take into account the angle a surface is viewed from, which can result in aliasing or bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultraviolet, and infrared light. Because light is an electromagnetic wave, other forms of electromagnetic radiation such as X-rays, microwaves, and radio waves exhibit similar properties. Most optical phenomena can be accounted for by using the classical electromagnetic description of light. Complete electromagnetic descriptions of light are, however, often difficult to apply in practice. Practical optics is usually done using simplified models. The most common of these, geometric optics, treats light as a collection of rays that travel in straight lines and bend when they pass through or reflect from surfaces. Physical optics is a more comprehensive model of light, which includes wave effects such as diffraction and interference that cannot be ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyotropic
A liquid crystalline mesophase is called lyotropic (a portmanteau of lyo- "dissolve" and -tropic "change" ) if formed by dissolving an amphiphilic mesogen in a suitable solvent, under appropriate conditions of concentration, temperature and pressure. A mixture of soap and water is an everyday example of a lyotropic liquid crystal. Historically, the term was used to describe the common behavior of materials composed of amphiphilic molecules upon the addition of a solvent. Such molecules comprise a water-loving hydrophilic head-group (which may be ionic or non-ionic) attached to a water-hating, hydrophobic group. The micro-phase segregation of two incompatible components on a nanometer scale results in different type of solvent-induced extended anisotropic arrangement, depending on the volume balances between the hydrophilic part and hydrophobic part. In turn, they generate the long-range order of the phases, with the solvent molecules filling the space around the compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LCP Structure
LCP may refer to: Science, medicine and technology *Large Combustion Plant, see Large Combustion Plant Directive *Le Chatelier's principle, equilibrium law in chemistry *Left Circular polarization, in radio communications * Legg–Calvé–Perthes syndrome, hip disorder *Licensed Clinical Psychologist, see Clinical psychology * Ligand close packing theory, in chemistry * Light compensation point, in biology *Linear complementarity problem, in mathematical optimisation *Link Control Protocol, in computer networking *Liquid Crystal Polymer, a kind of polymer *Liverpool Care Pathway for the Dying Patient, care guidance for dying hospital patients *Living cationic polymerization, a process in chemistry * Locking Compression Plate, an implant aiding the healing of a bone fracture * Long-chain polyunsaturated fatty acid * Longest Common Prefix array, in computer science Organisations * Latvijas Centrālās Padomes, Latvian Central Council *Lebanese Communist Party *Liberal and Country Par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
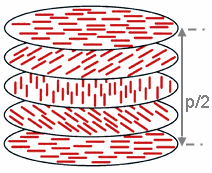
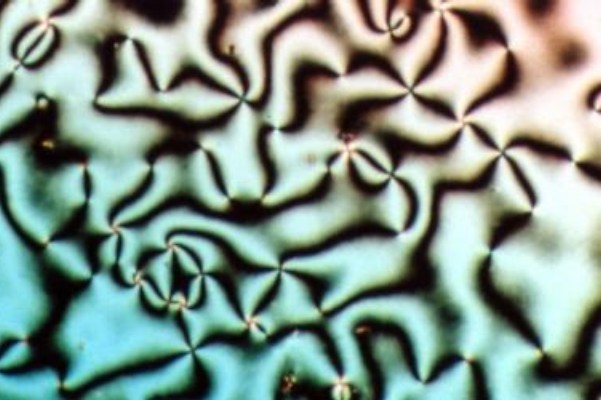
Cholesteric Liquid Crystal
A cholesteric liquid-crystal display (ChLCD) is a display containing a liquid crystal with a Helix, helical structure and which is therefore Chirality (chemistry), chiral. Cholesteric liquid crystals are also known as ''Liquid crystal#Chiral phases, chiral nematic liquid crystals''. They organize in layers with no positional ordering within layers, but a director axis which varies with layers. The variation of the director axis tends to be periodic in nature. The period of this variation (the distance over which a full rotation of 360° is completed) is known as the pitch, p. This pitch determines the wavelength of light which is reflected (Bragg reflection, Bragg Reflection). The technology is characterized by stable states i.e. focal conic state (dark state) and planar state (bright state). Displays based on this technology are called “bistable” and don’t need any power to maintain the information (zero power). Because of the reflective nature of the ChLCD, these displays ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesophase
In chemistry and chemical physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially ordered structure in a mesophase. Further, biological structures such as the lipid bilayers of cell membranes are examples of mesophases. Georges Friedel (1922) called attention to the "mesomorphic states of matter" in his scientific assessment of observations of the so-called liquid crystals. Conventionally a crystal is solid, and crystallization converts liquid to solid. The oxymoron of the liquid crystal is resolved through the notion of mesophases. The observations noted an optic axis persisting in materials that had been melted and had begun to flow. The term ''liquid crystal'' persists as a colloquialism, but use of the term was criticized in 1993: In ''The Physics of Liquid Crystals''P.G. de Gennes & J. Prost (1993) ''The Physics of Liquid Crystals'', 2nd edition, Oxford University Press the mesophases are introduced from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |