|
Limewater
Limewater is the common name for a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater is clear and colorless, with a slight earthy smell and an astringent/bitter taste. It is basic in nature with a pH of 12.4. Limewater may be prepared by mixing calcium hydroxide (Ca(OH)2) with water and removing excess undissolved solute (e.g. by filtration). When excess calcium hydroxide is added (or when environmental conditions are altered, e.g. when its temperature is raised sufficiently), a milky solution results due to the homogeneous suspension of excess calcium hydroxide. This liquid has been known traditionally as milk of lime. Chemistry An experiment commonly used to demonstrate the interaction of CO2 and Ca(OH)2 is as follows. Resultant carbon dioxide passed through limewater in the right tube, producing a milky solution due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limewater
Limewater is the common name for a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater is clear and colorless, with a slight earthy smell and an astringent/bitter taste. It is basic in nature with a pH of 12.4. Limewater may be prepared by mixing calcium hydroxide (Ca(OH)2) with water and removing excess undissolved solute (e.g. by filtration). When excess calcium hydroxide is added (or when environmental conditions are altered, e.g. when its temperature is raised sufficiently), a milky solution results due to the homogeneous suspension of excess calcium hydroxide. This liquid has been known traditionally as milk of lime. Chemistry An experiment commonly used to demonstrate the interaction of CO2 and Ca(OH)2 is as follows. Resultant carbon dioxide passed through limewater in the right tube, producing a milky solution due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persimmon
The persimmon is the edible fruit of a number of species of trees in the genus ''Diospyros''. The most widely cultivated of these is the Oriental persimmon, ''Diospyros kaki'' ''Diospyros'' is in the family Ebenaceae, and a number of non-persimmon species of the genus are grown for ebony timber. In 2019, China produced 75% of the world total of persimmons. Description Like the tomato, persimmons are not commonly considered to be berries, but Morphology (biology), morphologically the fruit is in fact a berry (botany), berry. The tree ''Diospyros kaki'' is the most widely cultivated species of persimmon. Typically the tree reaches in height and is round-topped. It usually stands erect, but sometimes can be crooked or have a willowy appearance. The leaves are long, and are Glossary of leaf morphology#oblong, oblong in shape with brown-hairy Petiole (botany), petioles in length. They are leathery and glossy on the upper surface, brown and silky underneath. The leaves are dec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lime Softening
Lime softening (also known as lime buttering, lime-soda treatment, or Clark's process) is a type of water treatment used for water softening, which uses the addition of limewater (calcium hydroxide) to remove hardness (deposits of calcium and magnesium salts) by precipitation. The process is also effective at removing a variety of microorganisms and dissolved organic matter by flocculation. History Lime softening was first used in 1841 to treat Thames River water. The process expanded in use as the other benefits of the process was discovered. Lime softening greatly expanded in use during the early 1900s as industrial water use expanded. Lime softening provides soft water that can, in some cases, be used more effectively for heat transfer and various other industrial uses. Chemistry As lime in the form of limewater is added to raw water, the pH is raised and the equilibrium of carbonate species in the water is shifted. Dissolved carbon dioxide (CO2) is changed into bicarbonate (H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corn Tortillas
In North America, a corn tortilla or just tortilla (, ) is a type of thin, unleavened flatbread, made from hominy, that is the whole kernels of maize treated with alkali to improve their nutrition in a process called nixtamalization. A simple dough made of ground, dried hominy, salt and water is then formed into flat discs and cooked on a very hot surface, generally an iron griddle called a comal. A similar flatbread from South America, called an ''arepa'' (though ''arepas'' are made with ground maize, not hominy, and are typically much thicker than tortillas), predates the arrival of Europeans to America, and was called ''tortilla'' by the Spanish from its resemblance to the traditional Spanish round, unleavened cakes and omelettes (originally made without potatoes, which are native to South America). The Aztecs and other Nahuatl-speakers call tortillas tlaxcalli (''Nahuatl Dictionary.'' (1997). Wired Humanities Project. University of Oregon. Retrieved August 29, 2012, frolink/r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
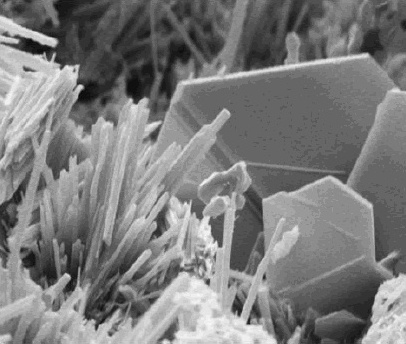
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nixtamalization
Nixtamalization () is a process for the preparation of corn, or other grain, in which the grain is soaked and cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), washed, and then hulled. The term can also refer to the removal via an alkali process of the pericarp from other grains such as sorghum. Nixtamalized corn has several benefits over unprocessed grain: It is more easily ground, its nutritional value is increased, flavor and aroma are improved, and mycotoxins are reduced by up to 97%–100% (for aflatoxins). Lime and ash are highly alkaline: the alkalinity helps the dissolution of hemicellulose, the major glue-like component of the maize cell walls, and loosens the hulls from the kernels and softens the maize. Corn's hemicellulose-bound niacin is converted to free niacin (a form of vitamin B3), making it available for absorption into the body, thus helping to prevent pellagra. Some of the corn oil is broken down in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate: :Ca(OH)2CO2->CaCO3H_2O The process of forming a carbonate is sometimes referred to as "carbonation", although this term usually refers to the process of dissolving carbon dioxide in water. Concrete Carbonatation is a slow process that occurs in concrete where lime (CaO, or Ca(OH)2( aq)) in the cement reacts with carbon dioxide (CO2) from the air and forms calcium carbonate. The water in the pores of Portland cement concrete is normally alkaline with a pH in the range of 12.5 to 13.5. This highly alkaline environment is one in which the steel rebar is passivated and is protected from corrosion. According to the Pourbaix diagram for iron, the metal is passive when the pH is above 9.5.{{cite web , url=http://www.corrosion-doctors.org/Thermo/ironE-pH.htm , title=Pourbaix diagram of iron , publisher=Corrosion-doctors.org , date= , accessdate=2009-10 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celts
The Celts (, see pronunciation for different usages) or Celtic peoples () are. "CELTS location: Greater Europe time period: Second millennium B.C.E. to present ancestry: Celtic a collection of Indo-European peoples. "The Celts, an ancient Indo-European people, reached the apogee of their influence and territorial expansion during the 4th century bc, extending across the length of Europe from Britain to Asia Minor."; . " e Celts, were Indo-Europeans, a fact that explains a certain compatibility between Celtic, Roman, and Germanic mythology."; . "The Celts and Germans were two Indo-European groups whose civilizations had some common characteristics."; . "Celts and Germans were of course derived from the same Indo-European stock."; . "Celt, also spelled Kelt, Latin Celta, plural Celtae, a member of an early Indo-European people who from the 2nd millennium bce to the 1st century bce spread over much of Europe."; in Europe and Anatolia, identified by their use of Celtic langua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astringency
An astringent (sometimes called adstringent) is a chemical that shrinks or constricts body tissues. The word derives from the Latin ''adstringere'', which means "to bind fast". Calamine lotion, witch hazel, and yerba mansa, a Californian plant, are astringents. Astringency, the dry, puckering or numbing mouthfeel caused by the tannins in unripe fruits, lets the fruit mature by deterring eating. Ripe fruits and fruit parts including blackthorn (sloe berries), ''Aronia'' chokeberry, chokecherry, bird cherry, rhubarb, quince and persimmon fruits (especially those which are unripe), banana skins (or unripe bananas), cashew fruits and acorns are astringent. Citrus fruits, like lemons, are somewhat astringent. Tannins, being a kind of polyphenol, bind salivary proteins and make them precipitate and aggregate, producing a rough, "sandpapery", or dry sensation in the mouth. The tannins in some teas, coffee, and red grape wines like Cabernet Sauvignon and Merlot produce mild astringenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. Although alkalinity is primarily a term used by oceanographers, it is also used by hydrologists to describe temporary hardness. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from rainfall or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and borders fourteen countries by land, the most of any country in the world, tied with Russia. Covering an area of approximately , it is the world's third largest country by total land area. The country consists of 22 provinces, five autonomous regions, four municipalities, and two Special Administrative Regions (Hong Kong and Macau). The national capital is Beijing, and the most populous city and financial center is Shanghai. Modern Chinese trace their origins to a cradle of civilization in the fertile basin of the Yellow River in the North China Plain. The semi-legendary Xia dynasty in the 21st century BCE and the well-attested Shang and Zhou dynasties developed a bureaucratic political system to serve hereditary monarchies, or dyna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2_expt.png)