|
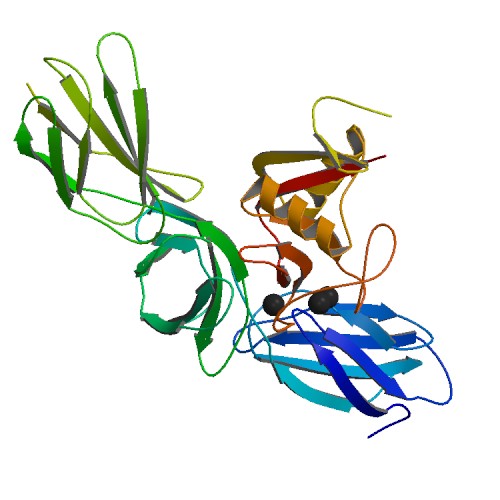
Lectican
Lecticans, also known as hyalectans, are a family of proteoglycans (a type protein that is attached to chains of negatively charged polysaccharides) that are components of the extracellular matrix. There are four members of the lectican family: aggrecan, brevican, neurocan, and versican. Lecticans interact with hyaluronic acid and tenascin-R to form a ternary complex. Tissue distribution Aggrecan is a major component of extracellular matrix in cartilage whereas versican is widely expressed in a number of connective tissues including those in vascular smooth muscle, skin epithelial cells, and the cells of central and peripheral nervous system. The expression of neurocan and brevican is largely restricted to neural tissues. Structure All four lecticans contain an N-terminal globular domain (G1 domain) that in turn contains an immunoglobulin V-set domain and a Link domain that binds hyaluronic acid; a long extended central domain (CS) that is modified with covalently atta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Versican
Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the ''VCAN'' gene. Versican is a large chondroitin sulfate proteoglycan with an apparent molecular mass of more than 1000kDa. In 1989, Zimmermann and Ruoslahti cloned and sequenced the core protein of fibroblast chondroitin sulfate proteoglycan. They designated it versican in recognition of its versatile modular structure. Versican belongs to the lectican protein family, with aggrecan (abundant in cartilage), brevican and neurocan (nervous system proteoglycans) as other members. Versican is also known as chondroitin sulfate proteoglycan core protein 2 or chondroitin sulfate proteoglycan 2 (CSPG2), and PG-M. Structure These proteoglycans share a homologous globular N-terminal, C-terminal, and glycosaminoglycan (GAG) binding regions. The N-terminal (G1) globular domain consists of Ig-like loop and two link modules, and has Hyaluronan (HA) binding properti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brevican
Brevican core protein is a protein that in humans is encoded by the ''BCAN'' gene. Brevican is a member of the lectican protein family. Brevican is localised to the surface of neurons in the brain. In melanocytic cells, BCAN gene expression may be regulated by MITF Microphthalmia-associated transcription factor also known as class E basic helix-loop-helix protein 32 or bHLHe32 is a protein that in humans is encoded by the ''MITF'' gene. MITF is a basic helix-loop-helix leucine zipper transcription factor .... References Further reading * * * * * * * * * * * * External links * C-type lectins Lecticans Extracellular matrix proteins {{gene-1-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aggrecan
Aggrecan (ACAN), also known as cartilage-specific proteoglycan core protein (CSPCP) or chondroitin sulfate proteoglycan 1, is a protein that in humans is encoded by the ''ACAN'' gene. This gene is a member of the lectican (chondroitin sulfate proteoglycan) family. The encoded protein is an integral part of the extracellular matrix in cartilagenous tissue and it withstands compression in cartilage. Aggrecan is a proteoglycan, or a protein modified with large carbohydrates; the human form of the protein is 2316 amino acids long and can be expressed in multiple isoforms due to alternative splicing. Aggrecan was named for its ability to form large aggregates in the cartilage tissue (a large aggregating proteoglycan). Structure Aggrecan is a high molecular weight (1x106 < M < 3x106) proteoglycan. It exhibits a bottlebrush structure, in which |
Neurocan
Neurocan core protein is a protein that in humans is encoded by the ''NCAN'' gene. Neurocan is a member of the lectican / chondroitin sulfate proteoglycan protein families and consists of neurocan core protein and chondroitin sulfate. It is thought to be involved in the modulation of cell adhesion and migration. Role in bipolar disorder Neurocan is a significant component of the extracellular matrix, and its levels are modulated by a variety of factors, but mice in which the NCAN gene has been knocked out show no easily observable defects in brain development or behavior. However, a genome-wide association study published in 2011 identified Neurocan as a susceptibility factor for bipolar disorder Bipolar disorder, previously known as manic depression, is a mental disorder characterized by periods of depression and periods of abnormally elevated mood that last from days to weeks each. If the elevated mood is severe or associated with .... A more comprehensive study pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenascin-R
Tenascin-R is a protein that in humans is encoded by the ''TNR'' gene. Function Tenascin-R (TNR) is an extracellular matrix protein expressed primarily in the central nervous system. It is a member of the tenascin (TN) gene family, which includes 4 genes in mammals: TNC (or hexabrachion), TNX is a Japanese holding company for various entertainment companies. Its subsidiaries include the talent agency Up-Front Promotion and Up-Front Works, a music production and sales company that manages such record labels as Zetima, Piccolo Town, ... (TNXB), TNW (also known as TNN) and TNR. The genes are expressed in distinct tissues at different times during embryonic development and are present in adult tissues. upplied by OMIMref name="entrez"/> References Further reading * * * * * * * * * * * Tenascins {{gene-1-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin V-set Domain
V-set domains are Ig-like domains resembling the antibody variable domain. V-set domains are found in diverse protein families, including immunoglobulin light and heavy chains; in several T-cell receptors such as CD2 (Cluster of Differentiation 2), CD4, CD80, and CD86; in myelin membrane adhesion molecules; in junctional adhesion molecules (JAM); in tyrosine-protein kinase receptors; and in the programmed cell death protein 1 (PD1). Subfamilies * Immunoglobulin V-set, subgroup * T-cell surface antigen CD2 Human proteins containing this domain ACAM; ACAN; ADAMTSL1; AGC1; AMICA1; BCAM; BCAN; BGP; BGPc; BT3.3; BTN1A1; BTN2A1; BTN2A2; BTN2A3; BTN3A1; BTN3A2; BTN3A3; BTNL2; BTNL3; BTNL8; BTNL9; C10orf54; C1orf32; C9orf94; CADM1; CADM2; CADM3; CADM4; CD2; CD226; CD274; CD276; CD300A; CD300C; CD300D; CD300E; CD300LB; CD300LF; CD300LG; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyaladherin
Hyaladherins, also known as hyaluronan-binding proteins, are proteins capable of binding to hyaluronic acid. Most hyaladherins belong to the Link module superfamily, including its main receptor CD44, hyalectans and TSG-6. In addition there is a diverse group of hyaladherins lacking a Link module; these include the receptor RHAMM, C1QBP (HABP1) and HABP2. The primary roles of hyaladherins are cell adhesion, structural support of the extracellular matrix (ECM) and cell signalling. Due to the role of aberrant hyaluronic acid synthesis and degradation in various cancers, hyaladherins, as well as hyaluronic acid, are considered a promising target for cancer therapy. See also *Hyaluronan synthase *Hyaluronidase Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid (HA). Karl Meyer classified these enzymes in 1971, into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyal ... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EGF-like Domain
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |