|
LG-120907
LG-120907 is a nonsteroidal antiandrogen (NSAA) of the quinoline group which was developed by Ligand Pharmaceuticals along with selective androgen receptor modulators (SARMs) like LG-121071 and was never marketed. The drug is a high-affinity antagonist of the androgen receptor (AR) with a Ki value of 26 nM and has been found to inhibit growth of the ventral prostate and seminal vesicles in male rats without increasing circulating levels of luteinizing hormone or testosterone. However, this tissue selectivity has not been assessed in humans. LG-120907 is orally active and shows greater oral potency than the aryl propionamide NSAA flutamide. The 7-fluoro derivative of LG-120907, LG-105, is also a potent NSAA, and appears to possess greater potency in comparison. Conversely, the 6- ethyl, 8-di desmethyl analogue of LG-120907, LG-121071, is a SARM with potent androgenic activity equivalent to that of dihydrotestosterone Dihydrotestosterone (DHT, 5α-dihydrotestoster ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LG-121071
LG121071 (or LGD-121071) is a selective androgen receptor modulator (SARM) developed by Ligand Pharmaceuticals that was first described in 1999 and was the first orally active nonsteroidal androgen to be discovered. It is a tricyclic quinolone derivative, structurally distinct from other nonsteroidal AR agonists like andarine and enobosarm (ostarine). The drug acts as a high-affinity full agonist of the androgen receptor (AR) (Ki = 17 nM), with a potency and efficacy that is said to be equivalent to that of dihydrotestosterone (DHT). Unlike testosterone, but similarly to DHT, LG121071 and other nonsteroidal androgens cannot be potentiated by 5α-reductase in androgenic tissues (nor aromatized into estrogenic metabolites), and for this reason, show tissue-selective androgenic effects. In accordance, they are said to possess full anabolic activity with reduced androgenic activity, similarly to anabolic-androgenic steroids. The ''in vitro'' metabolism Metabolism (, from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Antiandrogen
A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone. Medical uses NSAAs are used in clinical medicine for the following indications: * Prostate cancer in men * Androgen-dependent skin and hair conditions like acne, hirsutism, seborrhea, and pattern hair loss (androgenic alopecia) in women * Hyperandrogenism, such as due to polycystic ovary syndrome or congenital adrenal hyperplasia, in women * As a component of hormone therapy for transgender women * Precocious puberty in boys * Priapism in men Available forms Pharmacol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
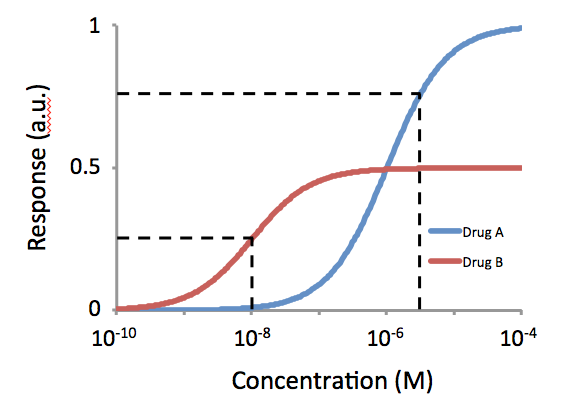
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Antiandrogens
A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically binding selectivity, selective and silent antagonist, full or silent antagonists of the androgen receptor (AR) and act by directly receptor antagonist, blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone. Medical uses NSAAs are used in clinical medicine for the following indications: * Prostate cancer in men * Androgen-dependent condition, Androgen-dependent dermatological condition, skin and hair conditions like acne, hirsutism, seborrhea, and pattern hair loss (androgenic alopecia) in women * Hyperandrogenism, such as due to polycystic ovary syndrome or congenital adrenal hyperplasia, in women * As a component of ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrotestosterone
Dihydrotestosterone (DHT, 5α-dihydrotestosterone, 5α-DHT, androstanolone or stanolone) is an endogenous androgen sex steroid and hormone. The enzyme 5α-reductase catalyzes the formation of DHT from testosterone in certain tissues including the prostate gland, seminal vesicles, epididymides, skin, hair follicles, liver, and brain. This enzyme mediates reduction of the C4-5 double bond of testosterone. Relative to testosterone, DHT is considerably more potent as an agonist of the androgen receptor (AR). In addition to its role as a natural hormone, DHT has been used as a medication, for instance in the treatment of low testosterone levels in men; for information on DHT as a medication, see the androstanolone article. Biological function DHT is biologically important for sexual differentiation of the male genitalia during embryogenesis, maturation of the penis and scrotum at puberty, growth of facial, body, and pubic hair, and development and maintenance of the pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes the embryological development of the primary male sex organs, and the development of male secondary sex characteristics at puberty. Androgens are synthesized in the testes, the ovaries, and the adrenal glands. Androgens increase in both males and females during puberty. The major androgen in males is testosterone. Dihydrotestosterone (DHT) and androstenedione are of equal importance in male development. DHT ''in utero'' causes differentiation of the penis, scrotum and prostate. In adulthood, DHT contributes to balding, prostate growth, and sebaceous gland activity. Although androgens are commonly thought of only as male sex hormones, females also have them, but at lower levels: they function in libido and sexual arousal. Also, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Analogue
A structural analog (analogue in modern traditional English; Commonwealth English), also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced with other atoms, groups, or substructures. A structural analog can be imagined to be formed, at least theoretically, from the other compound. Structural analogs are often isoelectronic. Despite a high chemical similarity, structural analogs are not necessarily functional analogs and can have very different physical, chemical, biochemical, or pharmacological properties. In drug discovery, either a large series of structural analogs of an initial lead compound are created and tested as part of a structure–activity relationship study or a database is screened for structural analogs of a lead compound. Chemical analogues of ille ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desmethyl
In chemical nomenclature, nor- is a prefix to name a structural analog that can be derived from a parent compound by the removal of one carbon atom along with the accompanying hydrogen atoms. The nor-compound can be derived by removal of a , , or CH group, or of a C atom. The "nor-" prefix also includes the elimination of a methylene bridge in a cyclic parent compound, followed by ring contraction. (The prefix "homo-" which indicates the next higher member in a homologous series, is usually limited to noncyclic carbons). ''"Since that time the meaning of the prefix has been generalized to denote the replacement of one or more methyl groups by H, or the disappearance of CH2 from a carbon chain"''. At present, the meaning is restricted to denote the removal of only one group from the parent structure, rather than the completely demethylated form of the parent compound. In literature, "nor" is sometimes called the "next lower homologue", although in this context Homology (chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl-containing compounds are generated by electrophilic ethylation, i.e. treatment of nucleophiles with sources of Et+. Triethyloxonium tetrafluoroborate t3OF4 is such a reagent. For good nucleophiles, less electrophilic reagents are employed, such as ethyl h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LG-105
The La Grande-1 (LG-1) is a hydroelectric power station on the La Grande River that is part of Hydro-Québec's James Bay Project. The station can generate 1,436 MW and was commissioned in 1994–1995. A run of the river generating station, it is one of only two generating stations of the James Bay Project that use a reservoir without any major water-level fluctuations (the Laforge-2 generating station is the other). Thus, the amount of electricity generated by the station depends almost entirely on the water-flow of the river, which is largely controlled by upstream reservoirs and generating stations. __NOTOC__ See also * List of largest power stations in Canada * List of electrical generating stations in Quebec * Reservoirs and dams in Canada * Hydro-Québec * James Bay Project * Chisasibi, Quebec Chisasibi ( cr, ᒋᓵᓰᐲ, translit=Cisâsîpî; meaning Great River) is a village on the eastern shore of James Bay, in the Eeyou Istchee equivalent territory (ET) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |