|
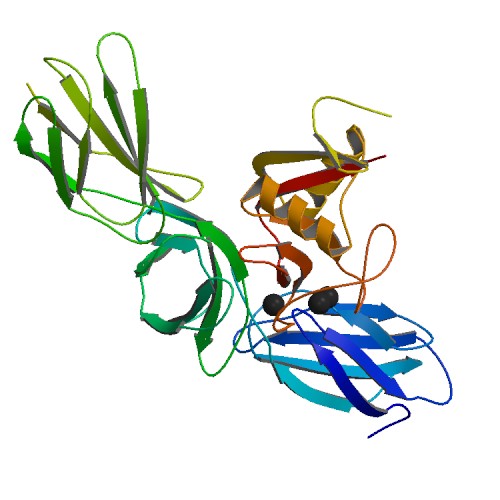
LEPRE1
Leprecan is a protein associated with osteogenesis imperfecta type VIII. Leprecan is part of a superfamily of 2OG-Fe(II) dioxygenase, along with DNA repair protein AlkB, and disease resistant EGL-9. The enzyme was found to be a type of hydroxylases used in the substrate formation of protein glycosylation. Activities Leprecan, a proteoglycan, has demonstrated prolyl hydroxylase activity; prolyl hydroxylases hydroxylate proline residues. Prolyl 3-hydroxylase 1, P3H1, forms a larger complex with CRTAP and cyclophilin B, CyPB, in the endoplasimic reticulum. The complex hydroxylates a single proline residue, Pro986, on collagen chains. Recessive forms of Osteogenesis Imperfecta are partly caused by a mutation in the ''LEPRE1'' gene that encodes prolyl 3-hydroxylase 1; malfunctioning prolyl 3-hydroxylase in leprecan leads to inappropriate collagen folding due to instability caused by the absence of hydroxyproline (2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteogenesis Imperfecta
Osteogenesis imperfecta (; OI), colloquially known as brittle bone disease, is a group of genetic disorders that all result in bones that break easily. The range of symptoms—on the skeleton as well as on the body's other organs—may be mild to severe. Symptoms found in various types of OI include whites of the eye (sclerae) that are blue instead, short stature, loose joints, hearing loss, breathing problems and problems with the teeth (dentinogenesis imperfecta). Potentially life-threatening complications, all of which become more common in more severe OI, include: tearing ( dissection) of the major arteries, such as the aorta; pulmonary valve insufficiency secondary to distortion of the ribcage; and basilar invagination. The underlying mechanism is usually a problem with connective tissue due to a lack of, or poorly formed, type I collagen. In more than 90% of cases, OI occurs due to mutations in the ''COL1A1'' or ''COL1A2'' genes. These mutations may be inherited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRTAP
Cartilage associated protein is a protein that in humans is encoded by the ''CRTAP'' gene. Function The protein encoded by this gene is similar to the chicken and mouse CRTAP genes. The encoded protein is a scaffolding protein that may influence the activity of at least one member of the cytohesin/ARNO family in response to specific cellular stimuli. Clinical significance Mutations in the CRTAP gene are associated with osteogenesis imperfecta Osteogenesis imperfecta (; OI), colloquially known as brittle bone disease, is a group of genetic disorders that all result in bones that break easily. The range of symptoms—on the skeleton as well as on the body's other organs—may b ..., types VII and IIB, a connective tissue disorder characterized by bone fragility and low bone mass. References Further reading * * * * * * * * * * * * * {{gene-3-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteogenesis Imperfecta
Osteogenesis imperfecta (; OI), colloquially known as brittle bone disease, is a group of genetic disorders that all result in bones that break easily. The range of symptoms—on the skeleton as well as on the body's other organs—may be mild to severe. Symptoms found in various types of OI include whites of the eye (sclerae) that are blue instead, short stature, loose joints, hearing loss, breathing problems and problems with the teeth (dentinogenesis imperfecta). Potentially life-threatening complications, all of which become more common in more severe OI, include: tearing ( dissection) of the major arteries, such as the aorta; pulmonary valve insufficiency secondary to distortion of the ribcage; and basilar invagination. The underlying mechanism is usually a problem with connective tissue due to a lack of, or poorly formed, type I collagen. In more than 90% of cases, OI occurs due to mutations in the ''COL1A1'' or ''COL1A2'' genes. These mutations may be inherited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclophilin
Cyclophilins (CYPs) are a family of proteins named after their ability to bind to ciclosporin (cyclosporin A), an immunosuppressant which is usually used to suppress rejection after internal organ transplants. They are found in all domains of life. These proteins have peptidyl prolyl isomerase activity, which catalyzes the isomerization of peptide bonds from ''trans'' form to ''cis'' form at proline residues and facilitates protein folding. Cyclophilin A is a cytosolic and highly abundant protein. The protein belongs to a family of isozymes, including cyclophilins B and C, and natural killer cell cyclophilin-related protein. Major isoforms have been found within single cells, including inside the Endoplasmic reticulum, and some are even secreted. Mammalian cyclophilins Human genes encoding proteins containing the cyclophilin domain include: * PPIA, PPIB, PPIC, PPID, PPIE, PPIF, PPIG, PPIH * PPIL1, PPIL2, PPIL3, PPIL4, PPIAL4, PPIL6 * PPWD1 Cyclophilin A Cyclophilin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
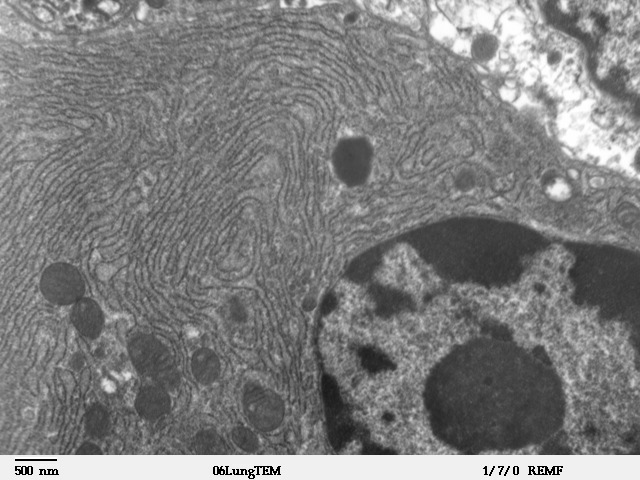
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Depending upon the degree of mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes one to two percent of muscle tissue and accounts for 6% of the weight of the skeletal muscle tissue. The fibroblast is the most common cell that crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |