|
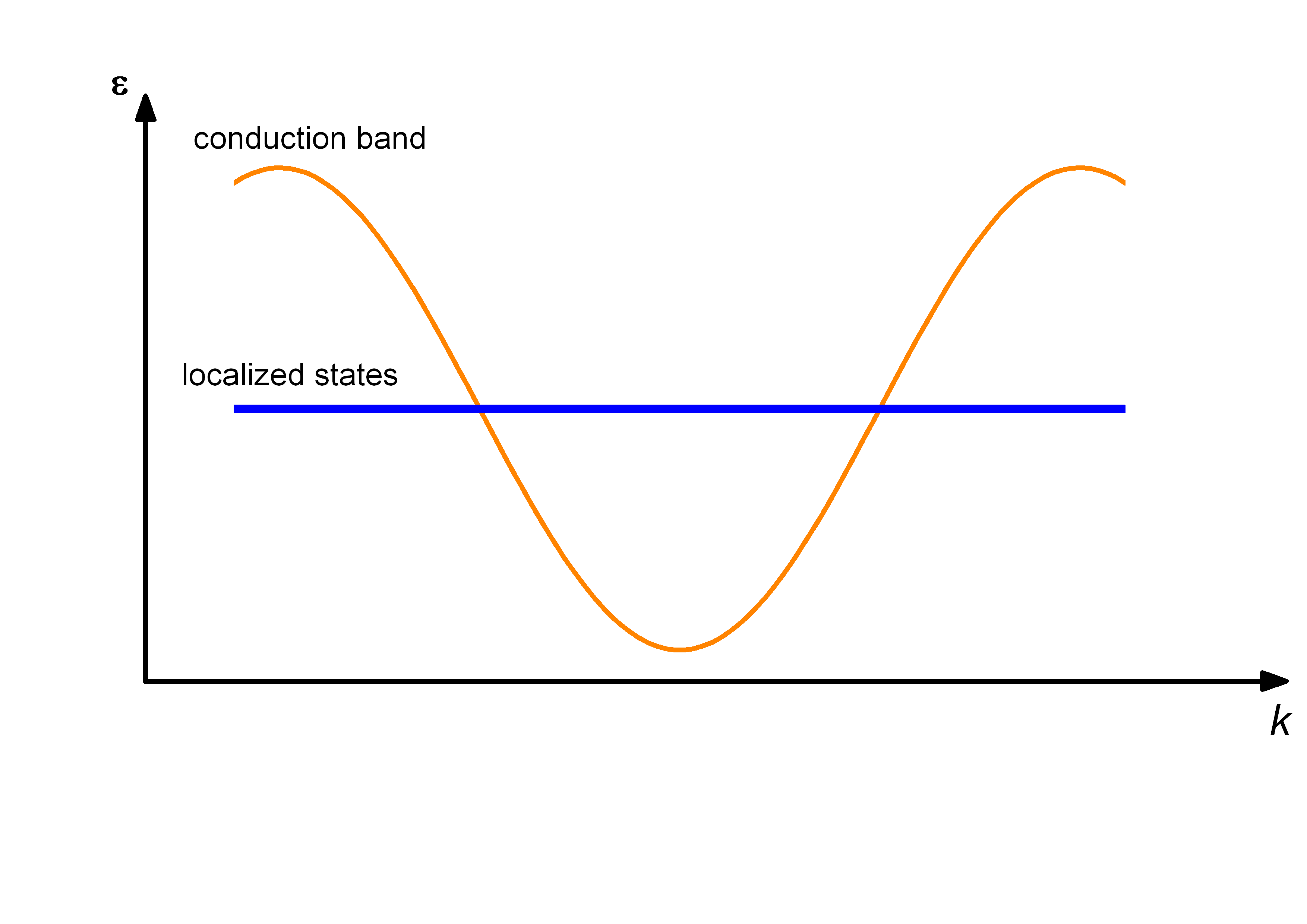
Kondo Insulator
In solid-state physics, Kondo insulators (also referred as Kondo semiconductors and heavy fermion semiconductors) are understood as materials with strongly correlated electrons, that open up a narrow band gap (in the order of 10 meV) at low temperatures with the chemical potential lying in the gap, whereas in heavy fermion materials the chemical potential is located in the conduction band. The band gap opens up at low temperatures due to hybridization of localized electrons (mostly f-electrons) with conduction electrons, a correlation effect known as the Kondo effect. As a consequence, a transition from metallic behavior to insulating behavior is seen in resistivity measurements. The band gap could be either direct or indirect. Most studied Kondo insulators are FeSi, Ce3Bi4Pt3, SmB6, YbB12, and CeNiSn, although there are over a dozen known Kondo insulators. Historical overview In 1969, Menth ''et al.'' found no magnetic ordering in SmB6 down to 0.35 K and a change from metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conduction Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angle-resolved Photoemission Spectroscopy
Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoelectric effect, in which an incoming photon of sufficient energy ejects an electron from the surface of a material. By directly measuring the kinetic energy and emission angle distributions of the emitted photoelectrons, the technique can map the electronic band structure and Fermi surfaces. ARPES is best suited for the study of one- or two-dimensional materials. It has been used by physicists to investigate high-temperature superconductors, graphene, Topological insulator, topological materials, quantum well states, and materials exhibiting charge density waves. ARPES systems consist of a monochromatic light source to deliver a narrow beam of photons, a sample holder connected to a manipulator used to position the sample of a material, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
F-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium Hexaboride
Samarium hexaboride (SmB6) is an intermediate-valence compound where samarium is present both as Sm2+ and Sm3+ ions at the ratio 3:7. It belongs to a class of Kondo insulators. At temperatures above 50 K its properties are typical of a Kondo metal, with metallic electrical conductivity characterized by strong electron scattering, whereas at low temperatures, it behaves as a non-magnetic insulator with a narrow band gap of about 4–14 meV. The cooling-induced metal-insulator transition in SmB6 is accompanied by a sharp increase in thermal conductivity, peaking at about 15 K. The reason for this increase is that electrons do not contribute to thermal conductivity at low temperatures, which is instead dominated by phonons. The decrease in electron concentration reduced the rate of electron-phonon scattering. Some research claims that it may be a topological insulator. Other researchers found no evidence of topological surface states. The increasing electrical resistance with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SmB6
Samarium hexaboride (SmB6) is an intermediate-valence compound where samarium is present both as Sm2+ and Sm3+ ions at the ratio 3:7. It belongs to a class of Kondo insulators. At temperatures above 50 K its properties are typical of a Kondo metal, with metallic electrical conductivity characterized by strong electron scattering, whereas at low temperatures, it behaves as a non-magnetic insulator with a narrow band gap of about 4–14 meV. The cooling-induced metal-insulator transition in SmB6 is accompanied by a sharp increase in thermal conductivity, peaking at about 15 K. The reason for this increase is that electrons do not contribute to thermal conductivity at low temperatures, which is instead dominated by phonons. The decrease in electron concentration reduced the rate of electron-phonon scattering. Some research claims that it may be a topological insulator. Other researchers found no evidence of topological surface states. The increasing electrical resistance with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct And Indirect Band Gaps
In semiconductor physics, the band gap of a semiconductor can be of two basic types, a direct band gap or an indirect band gap. The minimal-energy state in the conduction band and the maximal-energy state in the valence band are each characterized by a certain crystal momentum (k-vector) in the Brillouin zone. If the k-vectors are different, the material has an "indirect gap". The band gap is called "direct" if the crystal momentum of electrons and holes is the same in both the conduction band and the valence band; an electron can directly emit a photon. In an "indirect" gap, a photon cannot be emitted because the electron must pass through an intermediate state and transfer momentum to the crystal lattice. Examples of direct bandgap materials include amorphous silicon and some III-V materials such as InAs and GaAs. Indirect bandgap materials include crystalline silicon and Ge. Some III-V materials are indirect bandgap as well, for example AlSb. Implications for radiative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kondo Effect
In physics, the Kondo effect describes the scattering of conduction electrons in a metal due to magnetic impurities, resulting in a characteristic change i.e. a minimum in electrical resistivity with temperature. The cause of the effect was first explained by Jun Kondo, who applied third-order perturbation theory to the problem to account for scattering of s-orbital conduction electrons off d-orbital electrons localized at impurities ( Kondo model). Kondo's calculation predicted that the scattering rate and the resulting part of the resistivity should increase logarithmically as the temperature approaches 0 K. Experiments in the 1960s by Myriam Sarachik at Bell Laboratories provided the first data that confirmed the Kondo effect. Extended to a lattice of ''magnetic impurities'', the Kondo effect likely explains the formation of ''heavy fermions'' and ''Kondo insulators'' in intermetallic compounds, especially those involving rare earth elements such as cerium, praseodymium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
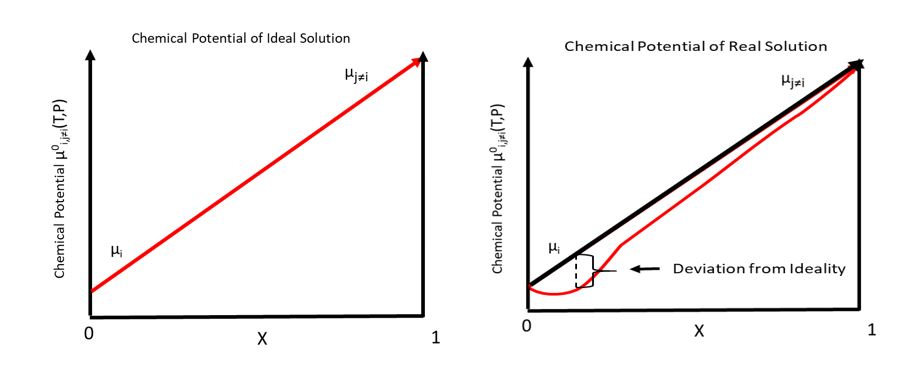
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hybridization
Hybridization (or hybridisation) may refer to: *Hybridization (biology), the process of combining different varieties of organisms to create a hybrid *Orbital hybridization, in chemistry, the mixing of atomic orbitals into new hybrid orbitals *Nucleic acid hybridization, the process of joining two complementary strands of nucleic acids - RNA, DNA or oligonucleotides *In evolutionary algorithms, the merging two or more optimization techniques into a single algorithm **Memetic algorithm, a common template for hybridization *In linguistics, the process of one variety blending with another variety *The alteration of a vehicle into a hybrid electric vehicle *In globalization theory, the ongoing blending of cultures *Hybridization in political election campaign communication, the combining of campaign techniques developed in different countries *In paleoanthropology, the hypothesis of Neanderthal and human hybridization See also *Hybrid (other) Hybrid may refer to: Science * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |