|
Ketobemidone Synthesis
Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway and Sweden and is used for severe pain. History Ketobemidone was first synthesized in 1942 by Eisleb and colleagues, at the laboratory of I.G. Farbenindustrie at Hoechst during the Second World War. The first study of it in humans was published in 1946, and it was introduced in clinical medicine shortly after. It was not in clinical use in the United States when the Controlled Substances Act 1970 was promulgated and was assigned to Schedule I with an ACSCN of 9628. As of 2013, no annual manufacturing quota was assigned by the DEA. Pfizer manufactures ketobemidone under the tradena ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
INCB
The International Narcotics Control Board (INCB) is an independent treaty body, one of the four treaty-mandated bodies under international drug control law (alongside the Commission on Narcotic Drugs, UNODC on behalf of the Secretary-General, and the WHO). The INCB is responsible for monitoring the control of substances pursuant to the three United Nations drug control conventions and for assisting Member States in their efforts to implement those conventions. It plays an important role in monitoring the production and trade of narcotics and psychotropics, as well as their availability for medical and scientific purposes, and in deciding which precursors should be regulated. History The Board has predecessors since the League of Nations. Following the 1909 Shanghai International Opium Commission, an International Opium Convention was adopted in 1925 and established the ''Permanent Central Opium Board'' (PCOB) which started its work in 1928. Later on, the 1931 Convention ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pethidine
Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a synthetic opioid analgesic, pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (piminodine, anileridine and others), the prodines (alphaprodine, MPPP, ''etc.''), bemidones (ketobemidone, etc.) and others more distant, including diphenoxylate and analogues. Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as a hydrochloride salt in tablets, as a syrup, or by intramuscular, Subcutaneous injection, subcutaneous, or intravenous injection. For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1975, 60% of doctors prescribed it for acu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextropropoxyphene
Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive (cough suppressant) and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia (pain relief) is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration. Dextropropoxyphene is sometimes combined with acetaminophen. Trade names include Darvocet-N, Di-Gesic, and Darvon with APAP (for dextropropoxyphene and paracetamol). The British approved name (i.e. the generic name of the active ingredient) of the paracetamol/dextropropoxyphene preparation is co-proxamol (sold under a vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoxymethylketobemidone
Acetoxymethylketobemidone (O-AMKD), is an opioid designer drug related to ketobemidone, with around the same potency as morphine. It was first identified in Germany in October 2020. See also * 2F-Viminol * 3-HO-PCP * 4-Fluoropethidine * Acetoxyketobemidone * Bucinnazine * Dipyanone * Etodesnitazene * Methylketobemidone * Nortilidine * O-Desmethyltramadol * Piperidylthiambutene * Propylketobemidone Propylketobemidone is an opioid analgesic that is an analogue of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the ... References Synthetic opioids 4-Phenylpiperidines Mu-opioid receptor agonists {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoxyketobemidone
Acetoxyketobemidone (O-Acetylketobemidone) is an opioid analgesic that is an acetylated derivative of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available. Nevertheless, acetoxyketobemidone is controlled as an ester of ketobemidone, which is included in Schedule I of the Single Convention on Narcotic Drugs of 1961. Presumably acetoxyketobemidone produces similar effects to ketobemidone and other opioids, such as analgesia and sedation, along with side effects such as nausea, itching, vomiting and respiratory depression Hypoventilation (also known as respiratory depression) occurs when ventilation is inadequate (''hypo'' meaning "below") to perform needed respiratory gas exchange. By definition it causes an increased concentration of carbon dioxi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketobemidone Synthesis
Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway and Sweden and is used for severe pain. History Ketobemidone was first synthesized in 1942 by Eisleb and colleagues, at the laboratory of I.G. Farbenindustrie at Hoechst during the Second World War. The first study of it in humans was published in 1946, and it was introduced in clinical medicine shortly after. It was not in clinical use in the United States when the Controlled Substances Act 1970 was promulgated and was assigned to Schedule I with an ACSCN of 9628. As of 2013, no annual manufacturing quota was assigned by the DEA. Pfizer manufactures ketobemidone under the tradena ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid has a p''K''a of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr almost uniquely participates in anti-Markovnikov hydrohalogenation of alkenes. The resulting 1-bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylmagnesium Bromide
Ethylmagnesium bromide is a Grignard reagent with formula C2H5MgBr. It is widely used in the laboratory synthesis of organic compounds. Reactions Apart from acting as the synthetic equivalent of an ethyl anion synthon for nucleophilic addition, ethylmagnesium bromide may be used as a strong base to deprotonate various substrates such as alkynes: :RC≡CH + EtMgBr → RC≡CMgBr + EtH In this application, ethylmagnesium bromide has been supplanted by the wide availability of organolithium reagents. Preparation Ethylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. It may be prepared in the normal manner of Grignard reagents — by reacting bromoethane with magnesium in diethyl ether Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(2-chloroethyl)methylamine
Chlormethine (INN, BAN), also known as mechlorethamine (USAN, USP), mustine, HN2, and (in post-Soviet states) embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance. Mechlorethamine belongs to the group of nitrogen mustard alkylating agents. Uses It has been derivatized into the estrogen analogue estramustine phosphate, used to treat prostate cancer. It can also be used in chemical warfare where it has the code-name HN2. This chemical is a form of nitrogen mustard gas and a powerful vesicant. Historically, some uses of mechlorethamine have included lymphoid ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
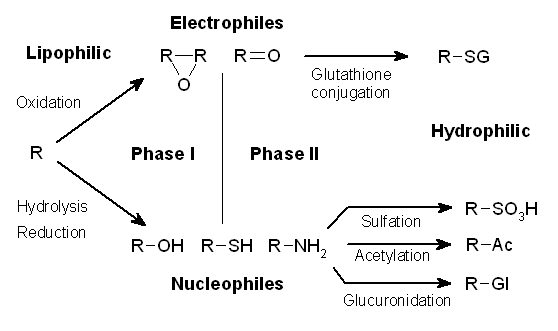
Xenobiotic Conjugation
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is called pharmacokinetics. The metabolism of pharmaceutical drugs is an important aspect of pharmacology and medicine. For example, the rate of metabolism determines the duration and intensity of a drug's pharmacologic action. Drug metabolism also affects mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |