|
Kerogen (musician)
Kerogen is solid, insoluble organic matter in sedimentary rocks. It consists of a variety of organic materials, including dead plants, algae, and other microorganisms, that have been compressed and heated by geological processes. Altogether kerogen is estimated to contain 1016 tons of carbon. This makes it the most abundant source of organic compounds on earth, exceeding the total organic content of living matter 10,000-fold. The type of kerogen present in a particular rock formation depends the type of organic material that was originally present. Kerogen can be classified by these origins: lacustrine (e.g., algal), marine (e.g., planktonic), and terrestrial (e.g., pollen and spores). The type of kerogen depends also on the degree of heat and pressure it has been subjected to, and the length of time the geological processes ran. The result is that a complex mixture of organic compounds reside in sedimentary rocks, serving as the precursor for the formation of hydrocarbons s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Matter
Organic matter, organic material, or natural organic matter refers to the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come from the feces and remains of organisms such as plants and animals. Organic molecules can also be made by chemical reactions that do not involve life. Basic structures are created from cellulose, tannin, cutin, and lignin, along with other various proteins, lipids, and carbohydrates. Organic matter is very important in the movement of nutrients in the environment and plays a role in water retention on the surface of the planet. Formation Living organisms are composed of organic compounds. In life, they secrete or excrete organic material into their environment, shed body parts such as leaves and roots and after organisms die, their bodies are broken down by bacterial and fungal action. Larger molecules of organic matter can be formed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
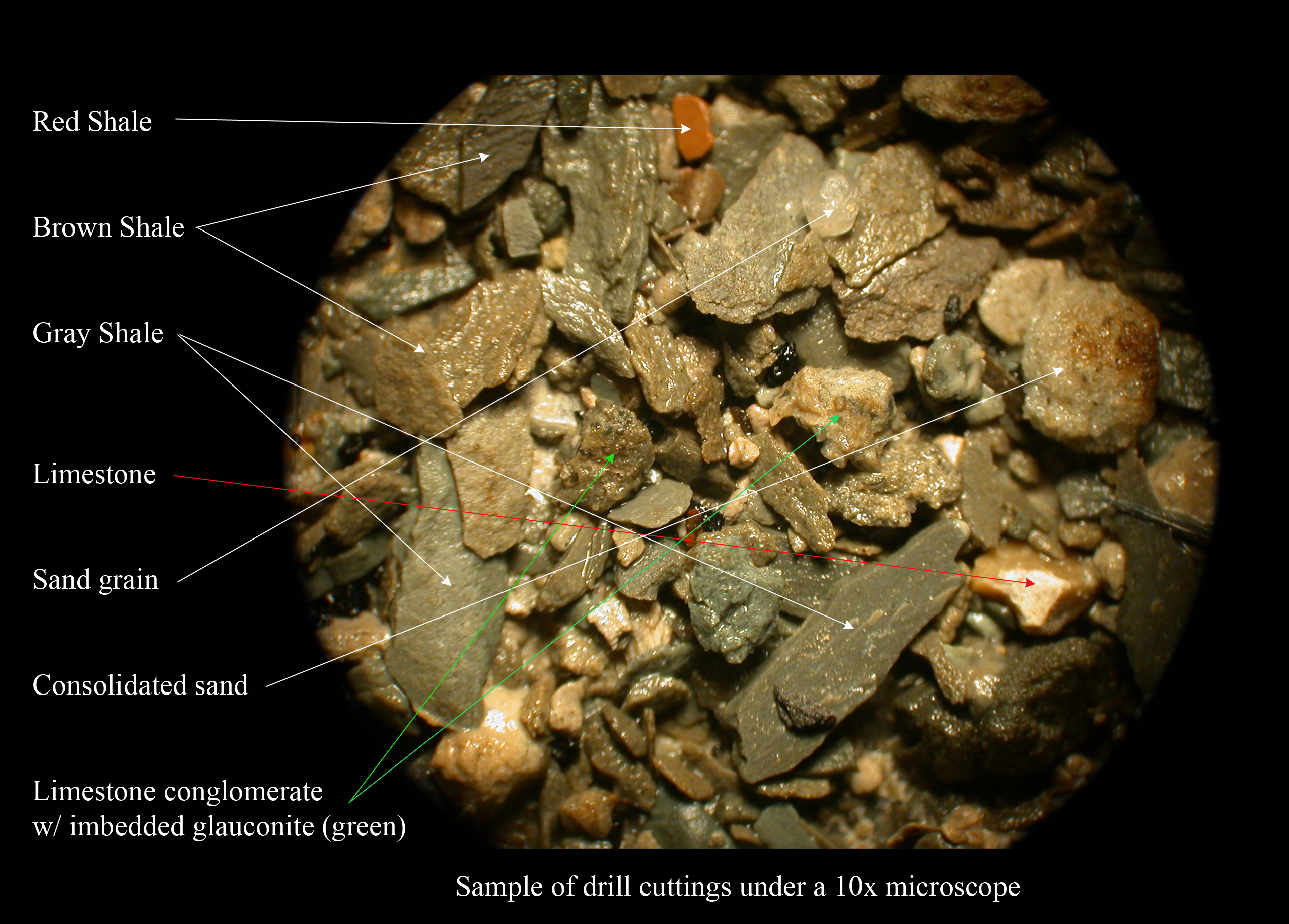
Shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers ( laminae) less than one centimeter in thickness. This property is called '' fissility''. Shale is the most common sedimentary rock. The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock, rather than in the more narrow sense of clay-rich fissile mudrock. Texture Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks into thin layers, often splintery and usually parallel to the otherwise indistinguishable beddin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geopolymers
Geopolymers are inorganic, typically ceramic, alumino-silicate forming long-range, covalently bonded, non-crystalline (amorphous) networks. Obsidian (volcanic glass) fragments are a component of some geopolymer blends. Commercially produced geopolymers may be used for fire- and heat-resistant coatings and adhesives, medicinal applications, high-temperature ceramics, new binders for fire-resistant fiber composites, toxic and radioactive waste encapsulation and new cements for concrete. The properties and uses of geopolymers are being explored in many scientific and industrial disciplines: modern inorganic chemistry, physical chemistry, colloid chemistry, mineralogy, geology, and in other types of engineering process technologies. The field of geopolymers is a part of polymer science, chemistry and technology that forms one of the major areas of materials science. Polymers are either organic material, i.e. carbon-based, or inorganic polymer, for example silicon-based. The organic pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide. The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids. Many variations of condensation reactions exist. Common examples include the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tucker M
Tucker may refer to: Places United States * Tucker, Arkansas * Tucker, Georgia * Tucker, Mississippi * Tucker, Missouri * Tucker, Utah, ghost town * Tucker County, West Virginia Outer space * Tucker (crater), a small lunar impact crater in the southern part of the Mare Smythii People * Tucker (given name), a page for people with the given name "Tucker" *Tucker (surname), a page for people with the last name "Tucker" *Tucker (American wrestler) * Tucker (Northern Irish wrestler) Art, entertainment, and media Fictional entities *Tucker, a Shetland pony in the film, ''Racing Stripes'' *Tucker Crowe, the fictional reclusive singer-songwriter in Nick Hornby's novel ''Juliet, Naked'' *Tucker Foley, one of the titular character's best friends on the animated series ''Danny Phantom'' *Tucker Jenkins, played by actor Todd Carty in the BBC television series ''Grange Hill'' and spin-off ''Tucker's Luck'' *Cameron Tucker, fictional character in the television series ''Modern Family'' *Tuck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name ''photosynthesis'', from the Greek ''phōs'' (), "light", and ''synthesis'' (), "putting together". Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth. Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centers that contain green chlorophyll (and other colored) pigments/chromoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrates
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may not be different from ''n''), which does not mean the H has covalent bonds with O (for example with , H has a covalent bond with C but not with O). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid). The term is most common in biochemistry, where it is a synonym of saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular weight) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopolymers
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose and alginate. Other examples of biopolymers include natural rubbers (polymers of isoprene), suberin and lignin (complex polyphenolic polymers), cutin and cutan (complex polymers of long-chain fatty acids) and melanin. In addition to their many essential roles in living organisms, biopolymers have applications in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plankton
Plankton are the diverse collection of organisms found in Hydrosphere, water (or atmosphere, air) that are unable to propel themselves against a Ocean current, current (or wind). The individual organisms constituting plankton are called plankters. In the ocean, they provide a crucial source of food to many small and large aquatic organisms, such as bivalves, fish and whales. Marine plankton include bacteria, archaea, algae, protozoa and drifting or floating animals that inhabit the saltwater of oceans and the brackish waters of estuaries. Freshwater plankton are similar to marine plankton, but are found in the freshwaters of lakes and rivers. Plankton are usually thought of as inhabiting water, but there are also airborne versions, the aeroplankton, that live part of their lives drifting in the atmosphere. These include plant spores, pollen and wind-scattered seeds, as well as microorganisms swept into the air from terrestrial dust storms and oceanic plankton swept into the air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |