|
KDELR1
KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1, also known as KDELR1, is a protein which in humans is encoded by the ''KDELR1'' gene. Function Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis- Golgi or a pre-Golgi compartment. Sorting of these proteins is dependent on a C-terminal tetrapeptide signal, usually lys- asp- glu- leu ( KDEL) in animal cells and his-asp-glu-leu ( HDEL) in ''S. cerevisiae''. This process is mediated by a receptor that recognizes and binds the tetrapeptide-containing protein and then returns it to the ER. In yeast, the sorting receptor is encoded by a single gene, ERD2, which is a seven-transmembrane protein. Unlike yeast, several human homologs of the ERD2 gene, constituting the KDEL receptor gene family, have been described. The protein encoded by this gene was the first member of the family to be identi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KDELR3
ER lumen protein retaining receptor 3 is a protein that in humans is encoded by the ''KDELR3'' gene. Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis-Golgi, or a pre-Golgi compartment. Sorting of these proteins is dependent on a C-terminal tetrapeptide signal, lys- asp- glu- leu ( KDEL) in animal cells and his-asp-glu-leu ( HDEL) in ''S. cerevisiae''. This process is mediated by a receptor that recognizes, and binds the tetrapeptide-containing protein, and returns it to the ER. In yeast, the sorting receptor encoded by a single gene, ERD2, is a seven-transmembrane protein. Unlike yeast, several human homologs of the ERD2 gene, constituting the KDEL receptor gene family, have been described. KDELR3 was the third member of the family to be identified, and it encodes a protein highly homologous to KDELR1 and KDELR2 proteins. Two transcript variants of KDELR3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KDELR2
ER lumen protein retaining receptor 2 is a protein that in humans is encoded by the ''KDELR2'' gene. Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis-Golgi, or a pre-Golgi compartment. Sorting of these proteins is dependent on a C-terminal tetrapeptide signal, lys- asp- glu- leu ( KDEL) in animal cells and his-asp-glu-leu ( HDEL) in ''S. cerevisiae''. This process is mediated by a receptor that recognizes, and binds the tetrapeptide-containing protein, and returns it to the ER. In yeast, the sorting receptor encoded by a single gene, ERD2, is a seven-transmembrane protein. Unlike yeast, several human homologs of the ERD2 gene, constituting the KDEL receptor gene family, have been described. KDELR2 was the second member of the family to be identified, and it encodes a protein which is 83% identical to the KDELR1 gene product. See also *KDELR1 *KDELR3 ER lum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KDEL (amino Acid Sequence)
KDEL is a target peptide sequence in mammals and plants located on the C-terminal end of the amino acid structure of a protein. The KDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported. A protein with a functional KDEL motif will be retrieved from the Golgi apparatus by retrograde transport to the ER lumen. It also targets proteins from other locations (such as the cytoplasm) to the ER. Proteins can only leave the ER after this sequence has been cleaved off. The abbreviation KDEL is formed by the corresponding letters to each amino acid. This letter system was defined by the IUPAC and IUBMB in 1983, and is as follows: *K—Lysine *D—Aspartic acid *E—Glutamic acid *L—Leucine Therefore, the KDEL sequence in three letter code is: Lysine, Lys-Aspartic acid, Asp-Glutamic acid, Glu-Leucine, Leu. The soluble resident protein will remain in the ER as long as it contains a KDEL signal seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
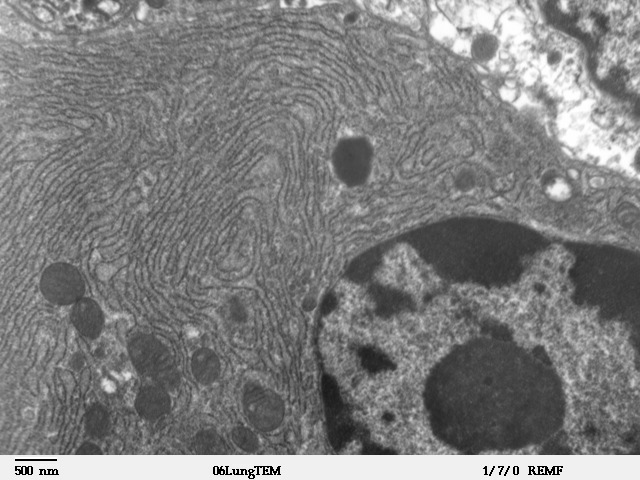
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARFGAP1
ADP-ribosylation factor GTPase-activating protein 1 is an enzyme that in humans is encoded by the ''ARFGAP1'' gene. Two transcript variants encoding different isoforms have been found for this gene. Function The protein encoded by this gene is a GTPase-activating protein (GAP) which associates with the Golgi apparatus and which interacts with ADP-ribosylation factor 1 (ARF1). The encoded protein promotes hydrolysis of ARF1-bound GTP and is required for the dissociation of coat proteins from Golgi-derived membranes and vesicles. Dissociation of the coat proteins is required for the fusion of these vesicles with target compartments. The activity of this protein is stimulated by phosphoinositides and inhibited by phosphatidylcholine. The protein has two amphipathic lipid packing sensor motifs (ALPS), that let the protein sense the curvature of the membrane (<30 nm) or lipid packing defects, and in this way evaluate if the vesicle is mature and ready for coat disassembly. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphocytes
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic adaptive immunity), and B cells (for humoral, antibody-driven adaptive immunity). They are the main type of cell found in lymph, which prompted the name "lymphocyte". Lymphocytes make up between 18% and 42% of circulating white blood cells. Types The three major types of lymphocyte are T cells, B cells and natural killer (NK) cells. Lymphocytes can be identified by their large nucleus. T cells and B cells T cells (thymus cells) and B cells ( bone marrow- or bursa-derived cells) are the major cellular components of the adaptive immune response. T cells are involved in cell-mediated immunity, whereas B cells are primarily responsible for humoral immunity (relating to antibodies). The function of T cells and B cells is to recognize specif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Transmembrane receptors include ligand-gated ion channels, G protein-coupled receptors, and enzyme-linked hormone receptors. Intracellular receptors are those found inside the cell, and include cytoplasmic receptors and nuclear receptors. A molecule that binds to a receptor is called a ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharomyces Cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular biology, molecular and cell biology, much like ''Escherichia coli'' as the model bacteria, bacterium. It is the microorganism behind the most common type of fermentation (biochemistry), fermentation. ''S. cerevisiae'' cells are round to ovoid, 5–10 micrometre, μm in diameter. It reproduces by budding. Many proteins important in human biology were first discovered by studying their Homology (biology), homologs in yeast; these proteins include cell cycle proteins, signaling proteins, and protein-processing enzymes. ''S. cerevisiae'' is currently the only yeast cell known to have Berkeley body, Berkeley bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HDEL (amino Acid Sequence)
HDEL is a target peptide sequence in plants and yeasts located on the C-terminal end of the amino acid structure of a protein. The HDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported. The similar sequence KDEL performs the same function in animals, while plants are known to utilize both KDEL and HDEL signaling sequences.{{cite journal, author1=Denecke J., author2=De Rycke R., author3=Botterman J., date=Jun 1992, title=Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope., journal=EMBO Journal, volume=11, issue=6, pages=2345–2355, doi=10.1002/j.1460-2075.1992.tb05294.x, pmc=556702, pmid=1376250 The abbreviation HDEL is as follows. * H—Histidine * D—Aspartic acid * E—Glutamic acid * L—Leucine Three letter code is: His His or HIS may refer to: Computing * Hightech Information System, a Hong Kong graphics card com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |