|
June Sutor
Dorothy June Sutor (6 June 1929 – 27 May 1990) was a New Zealand-born crystallographer who spent most of her research career in England. She was one of the first scientists to establish that hydrogen bonds could form to hydrogen atoms bonded to carbon atoms. She later worked in the laboratory of Kathleen Lonsdale on the characterisation and prevention of urinary calculi. Early life and education Sutor was born in New Zealand, in the Auckland suburb of Parnell, on 6 June 1929, the daughter of Victor Edward Sutor, a coach builder, and Cecilia Maud Sutor (née Craner). She was educated at St Cuthbert's College, and went on to study chemistry at Auckland University College. She graduated Master of Science with first-class honours in 1952 and, supervised by Frederick Llewellyn, she graduated with her first PhD in 1954. She published her first single-author ''Acta Crystallographica'' paper, ''The unit cell and space group of ethyl nitrolic acid'', whilst a student. In 1954, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auckland
Auckland (pronounced ) ( mi, Tāmaki Makaurau) is a large metropolitan city in the North Island of New Zealand. The List of New Zealand urban areas by population, most populous urban area in the country and the List of cities in Oceania by population, fifth largest city in Oceania, Auckland has an urban population of about It is located in the greater Auckland Region—the area governed by Auckland Council—which includes outlying rural areas and the islands of the Hauraki Gulf, and which has a total population of . While European New Zealanders, Europeans continue to make up the plurality of Auckland's population, the city became multicultural and Cosmopolitanism, cosmopolitan in the late-20th century, with Asian New Zealanders, Asians accounting for 31% of the city's population in 2018. Auckland has the fourth largest Foreign born, foreign-born population in the world, with 39% of its residents born overseas. With its large population of Pasifika New Zealanders, the city is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
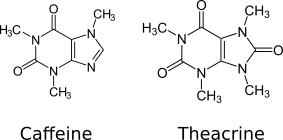
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine to the adenosine A1 receptor, which enhances release of the neurotransmitter acetylcholine. Caffeine has a three-dimensional structure similar to that of adenosine, which allows it to bind and block its receptors. Caffeine also increases cyclic AMP levels through nonselective inhibition of phosphodiesterase. Caffeine is a bitter, white crystalline purine, a methylxanthine alkaloid, and is chemically related to the adenine and guanine bases of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). It is found in the seeds, fruits, nuts, or leaves of a number of plants native to Africa, East Asia and South America, and helps to protect them against herbivores and from competition by preventing the germination of nearby seeds, as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theacrine
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu (''Theobroma grandiflorum'') and in a Chinese tea known as kucha () ('' Camellia assamica var. kucha''). It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine. In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway. Theacrine and caffeine are structurally similar. Safety Theacrine has demonstrated clinical safety and non-habituating effects in healthy humans over 8 weeks of daily use at up to 300 mg/day. Moreover, there was no evidence of tachyphylaxis that is typical of neuroactive agents such as caffeine and other stimulants. In animal studies, theacrine has an LD50 of 810 mg/kg, compared to 265 mg/kg for caffeine. See also * Liberine * Methylliberine * Theobromine * Theophylline Theophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C–H···O Interaction
In chemistry, a C–H···O interaction is occasionally described as a special type of weak hydrogen bond. These interactions frequently occur in the structures of important biomolecules like amino acids, proteins, sugars, DNA and RNA. History The C–H···O interaction was discovered in 1937 by Samuel Glasstone. Glasstone studied properties of mixtures of acetone with different halogenated derivatives of hydrocarbons and realized that dipole moments of these mixtures differ from dipole moments of pure substances. He explained this by establishing the concept of C–H···O interactions. The first crystallographic analysis of C-H ⋯O hydrogen bonds were published by June Sutor in 1962. Properties Similar to hydrogen bonds, a C–H···O interaction involves interactions of dipoles and therefore has directionality. The directionality of a C–H···O interaction is usually defined by the angle ''α'' between the С, Н and О atoms, and the distance ''d'' betw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Ionization
The degree of ionization (also known as ''ionization yield'' in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous solution, that are ionized. For electrolytes, it could be understood as a capacity of acid/base to ionize itself. A low degree of ionization is sometimes called ''partially ionized'' (also ''weakly ionized''), and a high degree of ionization as ''fully ionized''. However, fully ionized can also mean that an ion has no electrons left. Ionization refers to the process whereby an atom or molecule loses one or several electrons from its atomic orbital, or conversely gains an additional one, from an incoming free electron (electron attachment). In both cases, the atom or molecule ceases to be a neutral particle and becomes a charge carrier. If the species has lost one or several electrons, it becomes positively charged and is called a positive ion, or cation. On the contrary, if the species has gained one or several a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomenon resul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 greatest scientists of all time, and as of 2000, he was rated the 16th most important scientist in history. For his scientific work, Pauling was awarded the Nobel Prize in Chemistry in 1954. For his peace activism, he was awarded the Nobel Peace Prize in 1962. He is one of five people to have won more than one Nobel Prize (the others being Marie Curie, John Bardeen, Frederick Sanger and Karl Barry Sharpless). Of these, he is the only person to have been awarded two unshared Nobel Prizes, and one of two people to be awarded Nobel Prizes in different fields, the other being Marie Curie. Pauling was one of the founders of the fields of quantum chemistry and molecular biology. His contributions t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and location ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EDSAC
The Electronic Delay Storage Automatic Calculator (EDSAC) was an early British computer. Inspired by John von Neumann's seminal ''First Draft of a Report on the EDVAC'', the machine was constructed by Maurice Wilkes and his team at the University of Cambridge Mathematical Laboratory in England. EDSAC was the second electronic digital stored-program computer to go into regular service. Later the project was supported by J. Lyons & Co. Ltd., intending to develop a commercially applied computer and succeeding in Lyons' development of LEO I, based on the EDSAC design. Work on EDSAC started during 1947, and it ran its first programs on 6 May 1949, when it calculated a table of square numbers and a list of prime numbers. EDSAC was finally shut down on 11 July 1958, having been superseded by EDSAC 2, which remained in use until 1965. Technical overview Physical components As soon as EDSAC was operational, it began serving the university's research needs. It us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aaron Klug
Sir Aaron Klug (11 August 1926 – 20 November 2018) was a British biophysicist and chemist. He was a winner of the 1982 Nobel Prize in Chemistry for his development of crystallographic electron microscopy and his structural elucidation of biologically important nucleic acid-protein complexes. Early life and education Klug was born in Želva, in Lithuania, to Jewish parents Lazar, a cattleman, and Bella (née Silin) Klug, with whom he moved to South Africa at the age of two. He was educated at Durban High School. Paul de Kruif's 1926 book, '' Microbe Hunters'', aroused his interest in microbiology. He started to study microbiology, but then moved into physics and maths, graduating with a Bachelor of Science degree at the University of the Witwatersrand, in Johannesburg. He studied physics and obtained his Master of Science degree at the University of Cape Town. He was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851, which enabled him ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_p225_AUCKLAND%2C_NEW_ZEALAND.jpg)


.jpg)


