Partial Ionization on:
[Wikipedia]
[Google]
[Amazon]
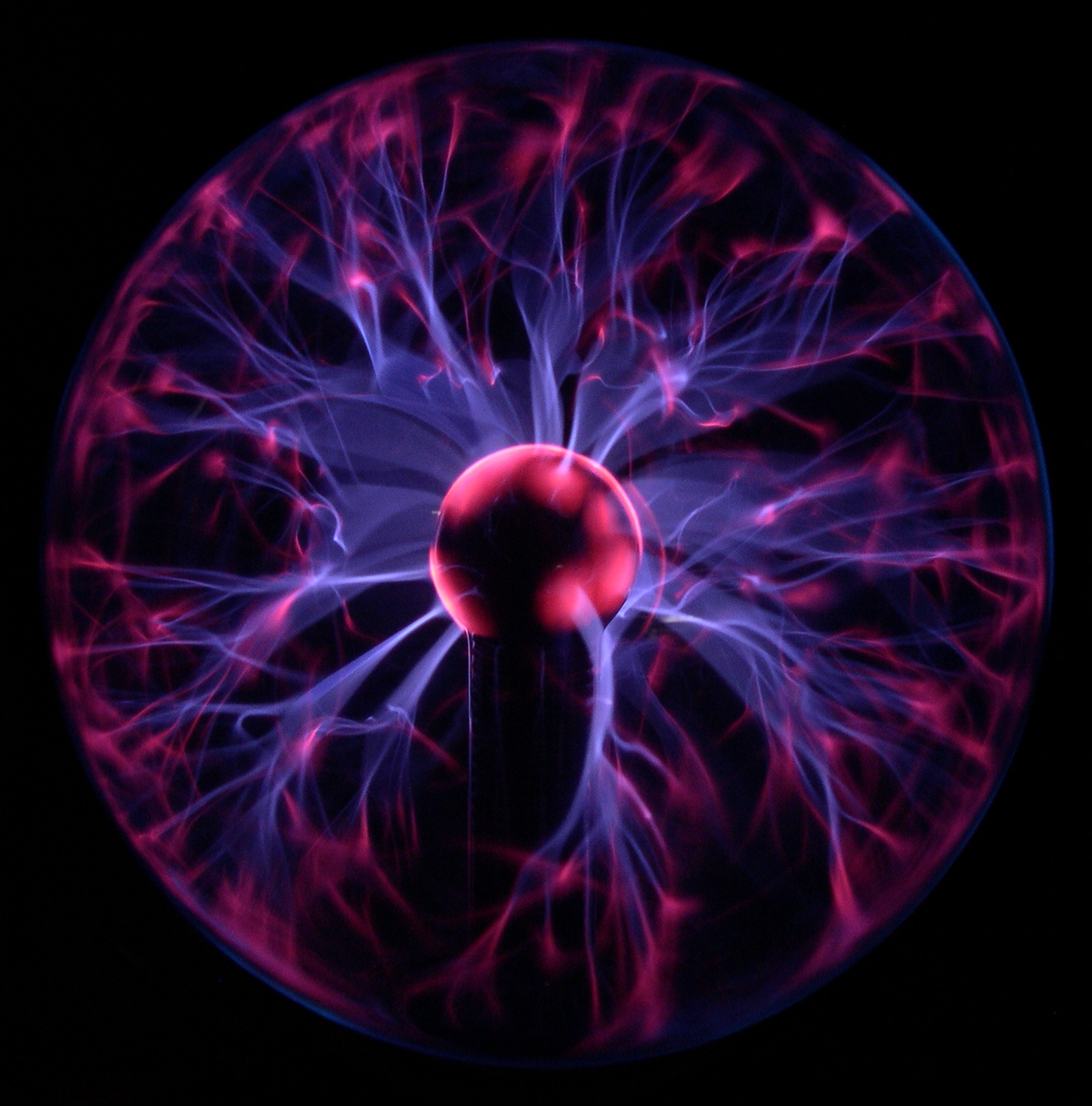 The degree of ionization (also known as ''ionization yield'' in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous solution, that are ionized. For
The degree of ionization (also known as ''ionization yield'' in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous solution, that are ionized. For
Oscillations in ionized gases
" ''Proc. Natl. Acad. Sci. U.S.'', vol. 14, p. 628, 1928 perhaps because it reminded him of a blood plasma.G. L. Rogoff, Ed., ''IEEE Transactions on Plasma Science'', vol. 19, p. 989, Dec. 1991. See extract at {{cite web , url=http://www.plasmacoalition.org/what.htm , title=Coalition for Plasma Science - What is a plasma? , accessdate=2006-05-24 , url-status=dead , archiveurl=https://web.archive.org/web/20060420130322/http://www.plasmacoalition.org/what.htm , archivedate=20 April 2006
 The degree of ionization (also known as ''ionization yield'' in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous solution, that are ionized. For
The degree of ionization (also known as ''ionization yield'' in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous solution, that are ionized. For electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s, it could be understood as a capacity of acid/base to ionize itself. A low degree of ionization is sometimes called ''partially ionized'' (also ''weakly ionized''), and a high degree of ionization as ''fully ionized''. However, fully ionized can also mean that an ion has no electrons left.
Ionization refers to the process whereby an atom or molecule loses one or several electrons from its atomic orbital, or conversely gains an additional one, from an incoming free
Free may refer to:
Concept
* Freedom, having the ability to do something, without having to obey anyone/anything
* Freethought, a position that beliefs should be formed only on the basis of logic, reason, and empiricism
* Emancipate, to procur ...
electron (electron attachment). In both cases, the atom or molecule ceases to be a neutral particle and becomes a charge carrier
In physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. The term is used ...
. If the species has lost one or several electrons, it becomes positively charged and is called a positive ion, or cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. On the contrary, if the species has gained one or several additional electrons, it becomes negatively charged
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
and is called a negative ion, or anion. Individual free electrons and ions in a plasma have very short lives typically inferior to the microsecond, as ionization and recombination, excitation
Excitation, excite, exciting, or excitement may refer to:
* Excitation (magnetic), provided with an electrical generator or alternator
* Excite Ballpark, located in San Jose, California
* Excite (web portal), web portal owned by IAC
* Electron exc ...
and relaxation are collective continuous processes.
Chemistry usage
The degree of dissociation ''α'' (also known as degree of ionization), is a way of representing the strength of an acid. It is defined as the ratio of the number of ionized molecules and the number of molecules dissolved in water. It can be represented as a decimal number or as a percentage. One can classify strong acids as those having ionization degrees above 30%, weak acids as those with ''α'' below 30%, and the rest as moderate acids, at a specified molar concentration.Physics usage
Inplasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
, the degree of ionization refers to the proportion of neutral particles that are ionized:
:
where is the ion density and the neutral density (in particles per cubic meter). It is a dimensionless number, sometimes expressed as a percentage.
When referred to an atom, "fully ionized" means that there are no bound electrons left, resulting in a bare nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
.
A particular case of fully ionized gases are very hot thermonuclear plasmas, such as plasmas artificially produced in nuclear explosions or naturally formed in our Sun and all stars in the universe. Regular stars largely contain hydrogen and helium that are fully ionized into protons (H+) and alpha-particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
(He2+).
History
Ionized matter was first identified in a discharge tube (or Crookes tube), and so described by Sir William Crookes in 1879 (he called it "radiant matter"). The nature of the Crookes tube " cathode ray" matter was subsequently identified by English physicist Sir J.J. Thomson in 1897, and dubbed "plasma" byIrving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publication is the 1919 art ...
in 1928,I. Langmuir,Oscillations in ionized gases
" ''Proc. Natl. Acad. Sci. U.S.'', vol. 14, p. 628, 1928 perhaps because it reminded him of a blood plasma.G. L. Rogoff, Ed., ''IEEE Transactions on Plasma Science'', vol. 19, p. 989, Dec. 1991. See extract at {{cite web , url=http://www.plasmacoalition.org/what.htm , title=Coalition for Plasma Science - What is a plasma? , accessdate=2006-05-24 , url-status=dead , archiveurl=https://web.archive.org/web/20060420130322/http://www.plasmacoalition.org/what.htm , archivedate=20 April 2006
See also
* List of plasma physics articlesFootnotes