|
Jpred
Jpred v.4 is the latest version of the JPred Protein Secondary Structure Prediction Server which provides predictions by the JNet algorithm, one of the most accurate methods for secondary structure prediction, that has existed since 1998 in different versions. In addition to protein secondary structure, JPred also makes predictions of solvent accessibility and coiled-coil regions. The JPred service runs up to 134 000 jobs per month and has carried out over 2 million predictions in total for users in 179 countries. JPred 2 The static HTML pages of JPred 2 are still available for reference. JPred 3 The JPred v3 followed on from previous versions of JPred developed and maintained by James Cuff and Jonathan Barber (see JPred References). This release added new functionality and fixed many bugs. The highlights are: * New, friendlier user interface * Retrained and optimised version of Jnet (v2) - mean secondary structure prediction accuracy of >81% * Batch submission of jobs * Better err ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSIPRED
PSI-blast based secondary structure PREDiction (PSIPRED) is a method used to investigate protein structure. It uses artificial neural network machine learning methods in its algorithm. It is a server-side program, featuring a website serving as a front-end interface, which can predict a protein's secondary structure (beta sheets, alpha helixes and coils) from the primary sequence. PSIPRED is available as a web service and as software. The software is distributed as source code, licensed technically as proprietary software. It allows modifying, but enforces freeware provisions by forbidding for-profit distribution of the software and its results. Secondary structure Secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids (DNA, RNA). It does not, however, describe specific atomic positions in three-dimensional space, which are considered to be the tertiary structure. Secondary structure can be formally defin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jalview
Jalview is a piece of bioinformatics software that is used to look at and edit multiple sequence alignments. The program was originally written by Michele Clamp whilst working in Geoff Barton's group at the University of Oxford and European Bioinformatics Institute (EBI). Jalview 2, a re-engineered version produced by Andrew Waterhouse and Jim Procter whilst working in Geoff Barton's group at the School of Life Sciences, University of Dundee, was released in 2005, and its development is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and Wellcome Trust. It is used widely by a variety of web servers (e.g. the EBI ClustalW server and the Pfam protein domain database) but is also available as a general purpose alignment editor. Jalview has a wide range of functions in addition to multiple sequence alignment generation, viewing and editing, including calculating phylogenetic trees and viewing molecular structures. Recent versions of Jalview include fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Secondary Structure
Protein secondary structure is the local spatial conformation of the polypeptide backbone excluding the side chains. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure elements typically spontaneously form as an intermediate before the protein folds into its three dimensional tertiary structure. Secondary structure is formally defined by the pattern of hydrogen bonds between the amino hydrogen and carboxyl oxygen atoms in the peptide backbone. Secondary structure may alternatively be defined based on the regular pattern of backbone dihedral angles in a particular region of the Ramachandran plot regardless of whether it has the correct hydrogen bonds. The concept of secondary structure was first introduced by Kaj Ulrik Linderstrøm-Lang at Stanford in 1952. Other types of biopolymers such as nucleic acids also possess characteristic secondary structures. Types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
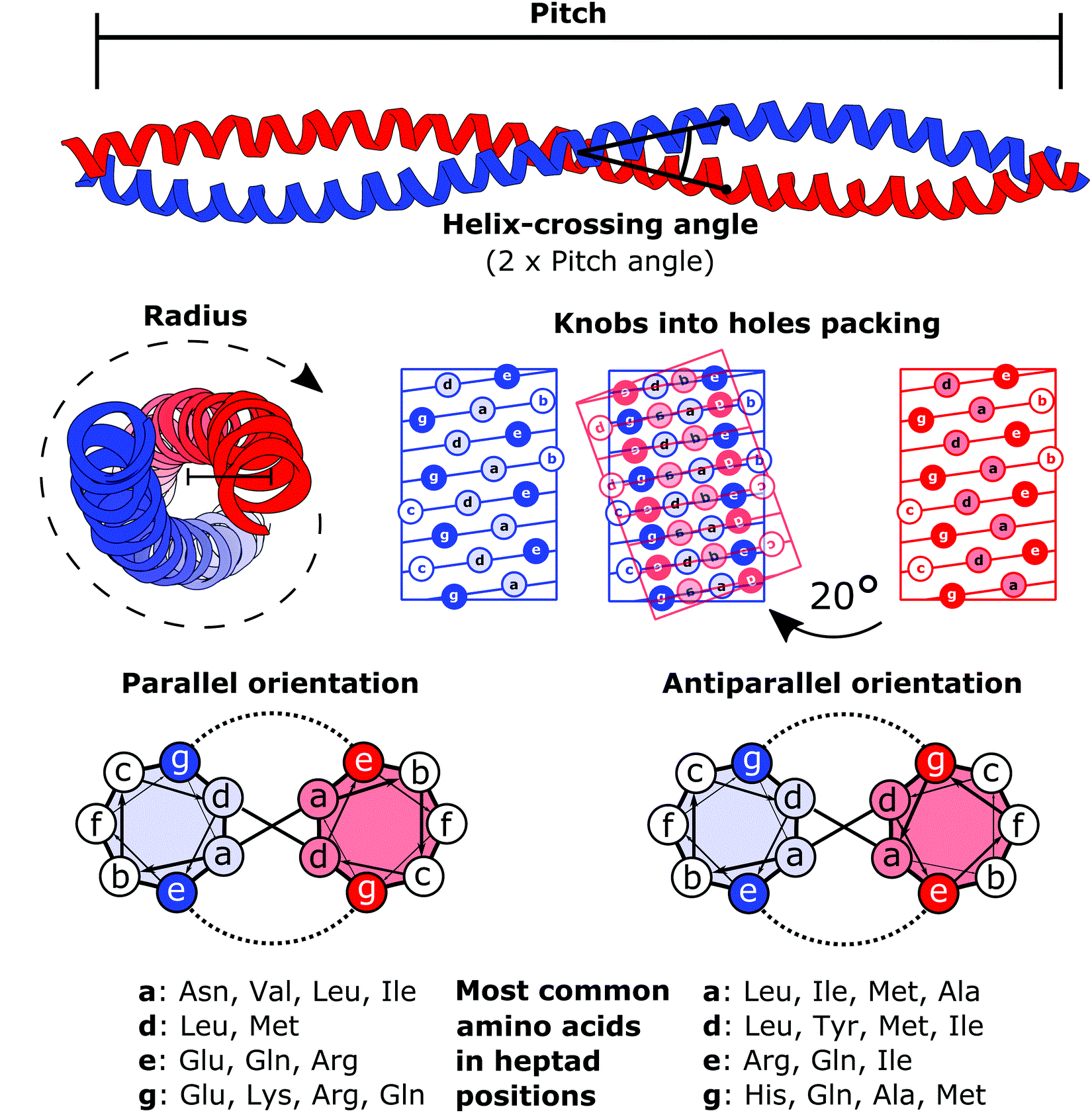
Coiled-coil
A coiled coil is a structural motif in proteins in which two to seven alpha-helices are coiled together like the strands of a rope. ( Dimers and trimers are the most common types.) They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where Cric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSI-BLAST
In bioinformatics, BLAST (basic local alignment search tool) is an algorithm and program for comparing Primary structure, primary biological sequence information, such as the amino acid, amino-acid sequences of proteins or the nucleotides of DNA sequence, DNA and/or RNA sequences. A BLAST search enables a researcher to compare a subject protein or nucleotide sequence (called a query) with a library or database of sequences, and identify database sequences that resemble the query sequence above a certain threshold. For example, following the discovery of a previously unknown gene in the Mus musculus, mouse, a scientist will typically perform a BLAST search of the human genome to see if humans carry a similar gene; BLAST will identify sequences in the human genome that resemble the mouse gene based on similarity of sequence. Background BLAST is one of the most widely used bioinformatics programs for sequence searching. It addresses a fundamental problem in bioinformatics research ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UniProt
UniProt is a freely accessible database of protein sequence and functional information, many entries being derived from genome sequencing projects. It contains a large amount of information about the biological function of proteins derived from the research literature. It is maintained by the UniProt consortium, which consists of several European bioinformatics organisations and a foundation from Washington, DC, USA. The UniProt consortium The UniProt consortium comprises the European Bioinformatics Institute (EBI), the Swiss Institute of Bioinformatics (SIB), and the Protein Information Resource (PIR). EBI, located at the Wellcome Trust Genome Campus in Hinxton, UK, hosts a large resource of bioinformatics databases and services. SIB, located in Geneva, Switzerland, maintains the ExPASy (Expert Protein Analysis System) servers that are a central resource for proteomics tools and databases. PIR, hosted by the National Biomedical Research Foundation (NBRF) at the George ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid that is four residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery In the early 1930s, William Astbury showed that there were d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of peptide, polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformational isomerism, conformation. The supramolecular association of β-sheets has been implicated in the formation of the Amyloid fibril, fibrils and Amyloid plaques, protein aggregates observed in amyloidosis, Alzheimer's disease and other Proteinopathy, proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neural Network
A neural network is a group of interconnected units called neurons that send signals to one another. Neurons can be either biological cells or signal pathways. While individual neurons are simple, many of them together in a network can perform complex tasks. There are two main types of neural networks. *In neuroscience, a '' biological neural network'' is a physical structure found in brains and complex nervous systems – a population of nerve cells connected by synapses. *In machine learning, an '' artificial neural network'' is a mathematical model used to approximate nonlinear functions. Artificial neural networks are used to solve artificial intelligence problems. In biology In the context of biology, a neural network is a population of biological neurons chemically connected to each other by synapses. A given neuron can be connected to hundreds of thousands of synapses. Each neuron sends and receives electrochemical signals called action potentials to its conne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Protein Structure Prediction Software
This list of protein structure prediction software summarizes notable used software tools in protein structure prediction, including homology modeling, protein threading, ''ab initio'' methods, secondary structure prediction, and transmembrane helix and signal peptide prediction. Software list Below is a list which separates programs according to the method used for structure prediction. Homology modeling Threading and fold recognition ''Ab initio'' structure prediction Secondary structure prediction Detailed list of programs can be found at List of protein secondary structure prediction programs See also * List of protein secondary structure prediction programs *Comparison of nucleic acid simulation software *List of software for molecular mechanics modeling *Molecular design software * Protein design *AlphaFold AlphaFold is an artificial intelligence (AI) program developed by DeepMind, a subsidiary of Alphabet, which performs predictions of protein struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |