|
JTE 7-31
JTE 7-31 is a selective cannabinoid receptor agonist invented by Japan Tobacco. It is a reasonably highly selective CB2 agonist, but still retains appreciable affinity at CB1, with a Ki of 0.088nM at CB2 vs 11nM at CB1. Legality JTE 7-31 is illegal in Alabama. See also * A-834,735 * JTE-907 * MDA-19 * N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide * S-444,823 * XLR-12 XLR-12 is an indole-based synthetic cannabinoid drug that was invented by Abbott Laboratories in 2006. It is an analogue of XLR-11 where the 5-fluoropentyl chain has been replaced with a 4,4,4-trifluorobutyl chain. XLR-12 is relatively highly s ... References External links Cannabinoids Japan Tobacco {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
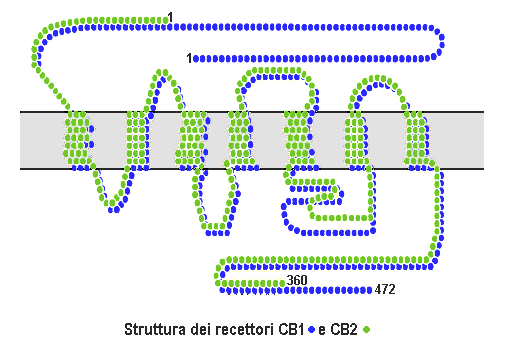
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Japan Tobacco
Hepburn: ''Nippon Senbai Kōsha'' , type = 1985-: Public (''kabushiki gaisha'') 1949-1985: Statutory corporation , traded_as = , industry = FoodTobacco , foundation = 1898 (as Imperial Japanese Tobacco Company)1 June 1949 (as Japan Tobacco and Salt Public Corporation)1 April 1985 (privatized) , founder = Government of Japan , location_city = 2-1, Toranomon, Minato, Tokyo, Japan , key_people = Mitsuomi Koizumi(President and CEO)Hiroshi Kimura(Chairman) , products = See below , revenue = (2012) , operating_income = (2012) , net_income = (2012) , assets = (2012) , equity = (2012) , num_employees = 48,529 (2011) , parent = Government of Japan (33.35% by law) , subsid = Japan Tobacco InternationalPT Karya Dibya Mahardhika (Indonesia)Mighty Corporation (Philippines)Donskoy Tabak Companies (Russian)Gallaher Group , homepage www.JT.com , abbreviated JT, is a cigarette manufacturing company. It is part of the Nikkei 225 and TOPIX Large70 indices. In 2009 the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 2
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 1
Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV). The primary endogenous agonist of the human CB1 receptor is anandamide. Structure The CB1 receptor shares the structure characteristic of all G-protein-coupled receptors, possessing seven transmembrane domains connected by three extracellular and three intracellular loops, an extracellular N-terminal tail, and an intracellular C-terminal tail. The receptor may exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently encount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alabama
(We dare defend our rights) , anthem = "Alabama (state song), Alabama" , image_map = Alabama in United States.svg , seat = Montgomery, Alabama, Montgomery , LargestCity = Huntsville, Alabama, Huntsville , LargestCounty = Baldwin County, Alabama, Baldwin County , LargestMetro = Birmingham metropolitan area, Alabama, Greater Birmingham , area_total_km2 = 135,765 , area_total_sq_mi = 52,419 , area_land_km2 = 131,426 , area_land_sq_mi = 50,744 , area_water_km2 = 4,338 , area_water_sq_mi = 1,675 , area_water_percent = 3.2 , area_rank = 30th , length_km = 531 , length_mi = 330 , width_km = 305 , width_mi = 190 , Latitude = 30°11' N to 35° N , Longitude = 84°53' W to 88°28' W , elevation_m = 150 , elevation_ft = 500 , elevation_max_m = 735.5 , elevation_max_ft = 2,413 , elevation_max_point = Mount Cheaha , elevation_min_m = 0 , elevation_min_ft = 0 , elevation_min_point = Gulf of Mexico , OfficialLang = English language, English , Languages = * English ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A-834,735
A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a ''K''i of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids, with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds, imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (''N''-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall. Legal status As of October 2015 A-834,735 is a controlled substance in China. See also * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JTE-907
JTE-907 is a drug used in scientific research that acts as a selective CB2 inverse agonist. It has antiinflammatory effects in animal studies, thought to be mediated by an interaction between the CB2 receptor and IgE Immunoglobulin E (IgE) is a type of antibody (or immunoglobulin (Ig) " isotype") that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains (ε chain) and two light chains, with the ε c .... See also * JTE 7-31 References {{cannabinoid-stub Cannabinoids Benzodioxoles 2-Quinolone ethers at the benzene ring Carboxamides Japan Tobacco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MDA-19
MDA-19 (also known as BZO-HEXOXIZID) is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not effect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9x higher than rat CB1 receptors. Discovery MDA-19 was first synthesized and studied in the late 2000s by researchers at the University of Texas M. D. Anderson Cancer Center. Pharmacology MDA-19 binds to human CB2 receptors at Ki = 43.3 +/- 10.3 nM and human CB1 receptors at Ki = 162.4 +/- 7.6 nM and functions as an agonist in human cannabinoid receptors but functions differently in rat cannabinoid receptors binding to rat canna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a ''K''i of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a ''K''i of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a ''K''i of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098). Chemically, it is closely related to another indole-3-carboxamide synthetic cannabinoid, Org 28611, but with a different cycloalkyl substitution on the carboxamide, and the cyclohexylmethyl group replaced by morpholinylethyl, as in JWH-200 or A-796,260. Early compounds such as these have subsequently led to the development of many related indole-3-carboxamide cannabinoid ligands. See also * A-834,735 * AB-00 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-444,823
S-444,823 is a drug developed by Shionogi which is a cannabinoid agonist. It was developed as an antipruritic, and has moderate selectivity for the CB2 subtype, having a CB2 affinity of 18nM, and 32x selectivity over the CB1 receptor. In animal studies it showed analgesic effects and strongly reduced itching responses, but without producing side effects such as sedation and catalepsy that are seen with centrally acting CB1 agonists. See also * JTE 7-31 * RQ-00202730 RQ-00202730 is a benzimidazole derived drug that acts as a potent and highly selective agonist for the CB2 cannabinoid receptor, with a Ki value of 19nM at CB2 and more than 4000x selectivity over CB1, though it also shows some activity as an anta ... * S-777,469 References Cannabinoids Nitrogen heterocycles Thiazoles {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
XLR-12
XLR-12 is an indole-based synthetic cannabinoid drug that was invented by Abbott Laboratories in 2006. It is an analogue of XLR-11 where the 5-fluoropentyl chain has been replaced with a 4,4,4-trifluorobutyl chain. XLR-12 is relatively highly selective for the CB2 receptor, with a Ki of 0.09 nM and 167x selectivity over the related CB1 receptor, however it still retains appreciable affinity for CB1 with a Ki of 15 nM. Legal status XLR-12 is illegal in Hungary and Japan. See also * UR-144 * FUB-144 * JTE 7-31 JTE 7-31 is a selective cannabinoid receptor agonist invented by Japan Tobacco. It is a reasonably highly selective CB2 agonist, but still retains appreciable affinity at CB1, with a Ki of 0.088nM at CB2 vs 11nM at CB1. Legality JTE 7-31 is il ... References Cannabinoids Designer drugs Tetramethylcyclopropanoylindoles {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |