|
Isotopes Of Technetium
Technetium (43Tc) is one of the two elements with that have no stable isotopes; the other such element is promethium. – Elements marked with a * have no stable isotope: 43, 61, and 83 and up. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission (there are an estimated grams of 99Tc per gram of pitchblende) or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc (half-life of 4.21 million years), 98Tc (half-life: 4.2 million years), and 99Tc (half-life: 211,100 years). Thirty-three other radioisotopes have been characterized with atomic masses ranging from 85Tc to 120Tc. Most of these have half-lives that are less than an hour; the exceptions are 93Tc (half-life: 2.75 hours), 94Tc (half-life: 4.883 hours), 95Tc (half-life: 20 hours), and 96Tc (half-life: 4.28 days). Technetium also has numero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only. Many of technetium's properties had been predicted by Dmitri Mendeleev before it was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
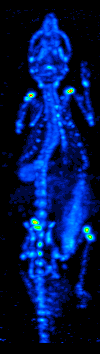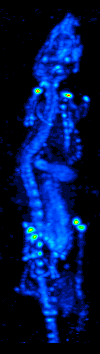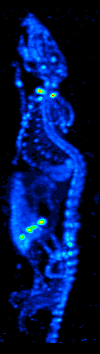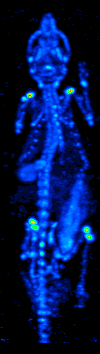
Technetium-99
Technetium-99 (99Tc) is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting beta particles, but no gamma rays. It is the most significant long-lived fission product of uranium fission, producing the largest fraction of the total long-lived radiation emissions of nuclear waste. Technetium-99 has a fission product yield of 6.0507% for thermal neutron fission of uranium-235. The metastable technetium-99m (99mTc) is a short-lived (half-life about 6 hours) nuclear isomer used in nuclear medicine, produced from molybdenum-99. It decays by isomeric transition to technetium-99, a desirable characteristic, since the very long half-life and type of decay of technetium-99 imposes little further radiation burden on the body. Radiation The weak beta emission is stopped by the walls of laboratory glassware. Soft X-rays are emitted when the beta particles are stopped, but as long as the body is kept more than 30 cm away these should p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
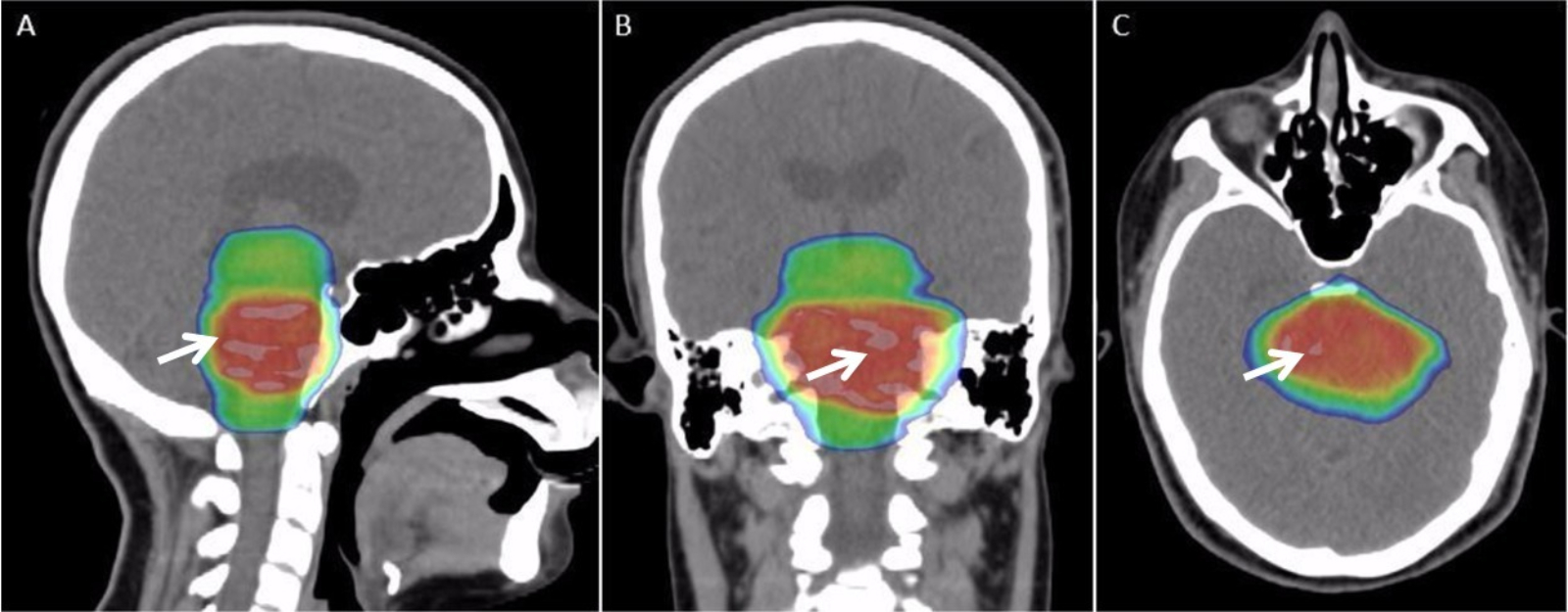
Radiation Therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizing radiation works by damaging the DNA of cancerous tissue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Generator
Neutron generators are neutron source devices which contain compact linear particle accelerators and that produce neutrons by fusing isotopes of hydrogen together. The fusion reactions take place in these devices by accelerating either deuterium, tritium, or a mixture of these two isotopes into a metal hydride target which also contains deuterium, tritium or a mixture of these isotopes. Fusion of deuterium atoms (D + D) results in the formation of a helium-3 ion and a neutron with a kinetic energy of approximately 2.5 MeV. Fusion of a deuterium and a tritium atom (D + T) results in the formation of a helium-4 ion and a neutron with a kinetic energy of approximately 14.1 MeV. Neutron generators have applications in medicine, security, and materials analysis. The basic concept was first developed by Ernest Rutherford's team in the Cavendish Laboratory in the early 1930s. Using a linear accelerator driven by a Cockcroft–Walton generator, Mark Oliphant led an experiment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D-D Fusion
Fusion power is a proposed form of power generation that would generate electricity by using heat from nuclear fusion reactions. In a fusion process, two lighter atomic nuclei combine to form a heavier nucleus, while releasing energy. Devices designed to harness this energy are known as fusion reactors. Research into fusion reactors began in the 1940s, but as of 2022, only one design, an inertial confinement laser-driven fusion machine at the US National Ignition Facility, has conclusively produced a positive fusion energy gain factor, i.e. more power output than input. Fusion processes require fuel and a confined environment with sufficient temperature, pressure, and confinement time to create a plasma in which fusion can occur. The combination of these figures that results in a power-producing system is known as the Lawson criterion. In stars, the most common fuel is hydrogen, and gravity provides extremely long confinement times that reach the conditions needed for fusion en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
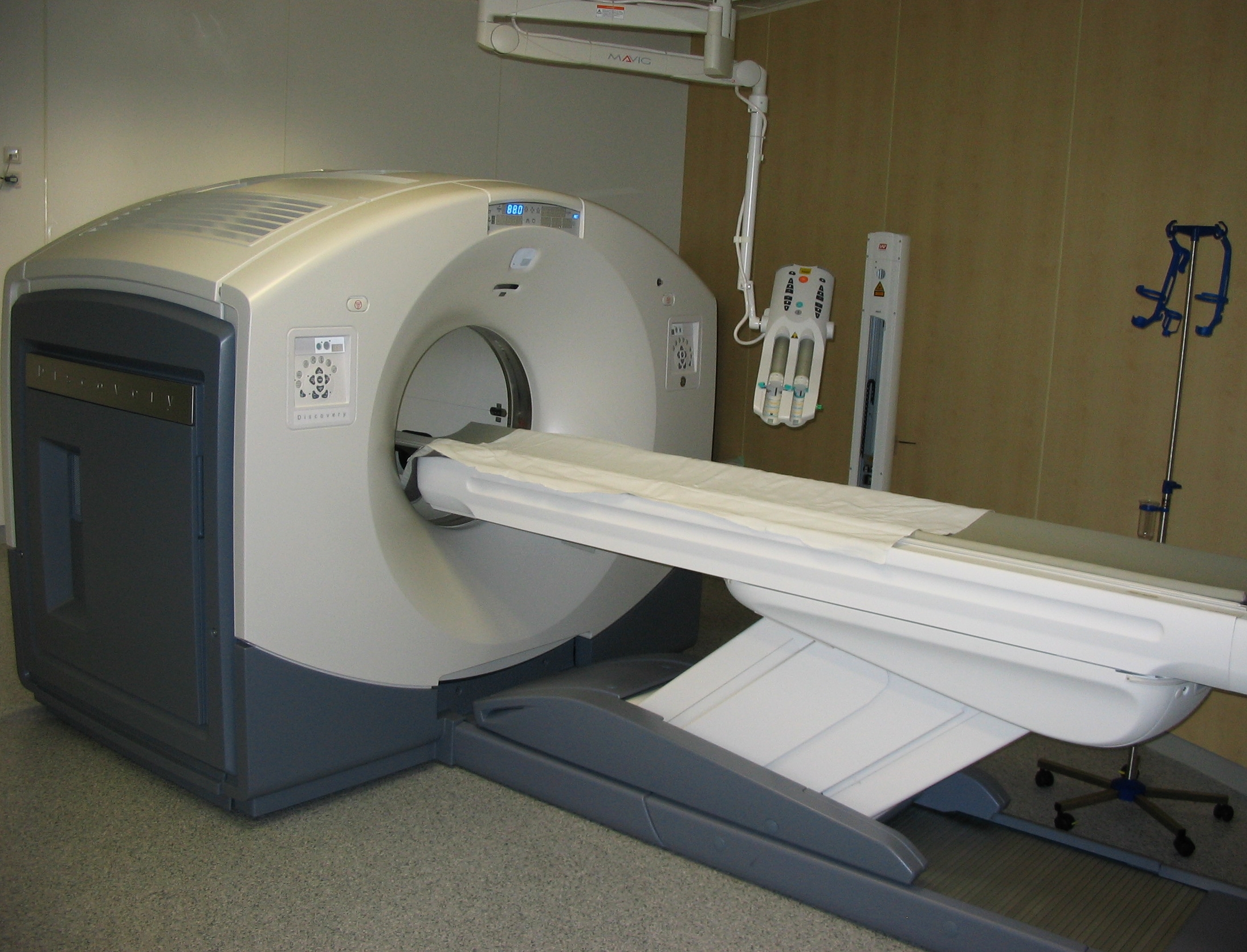
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
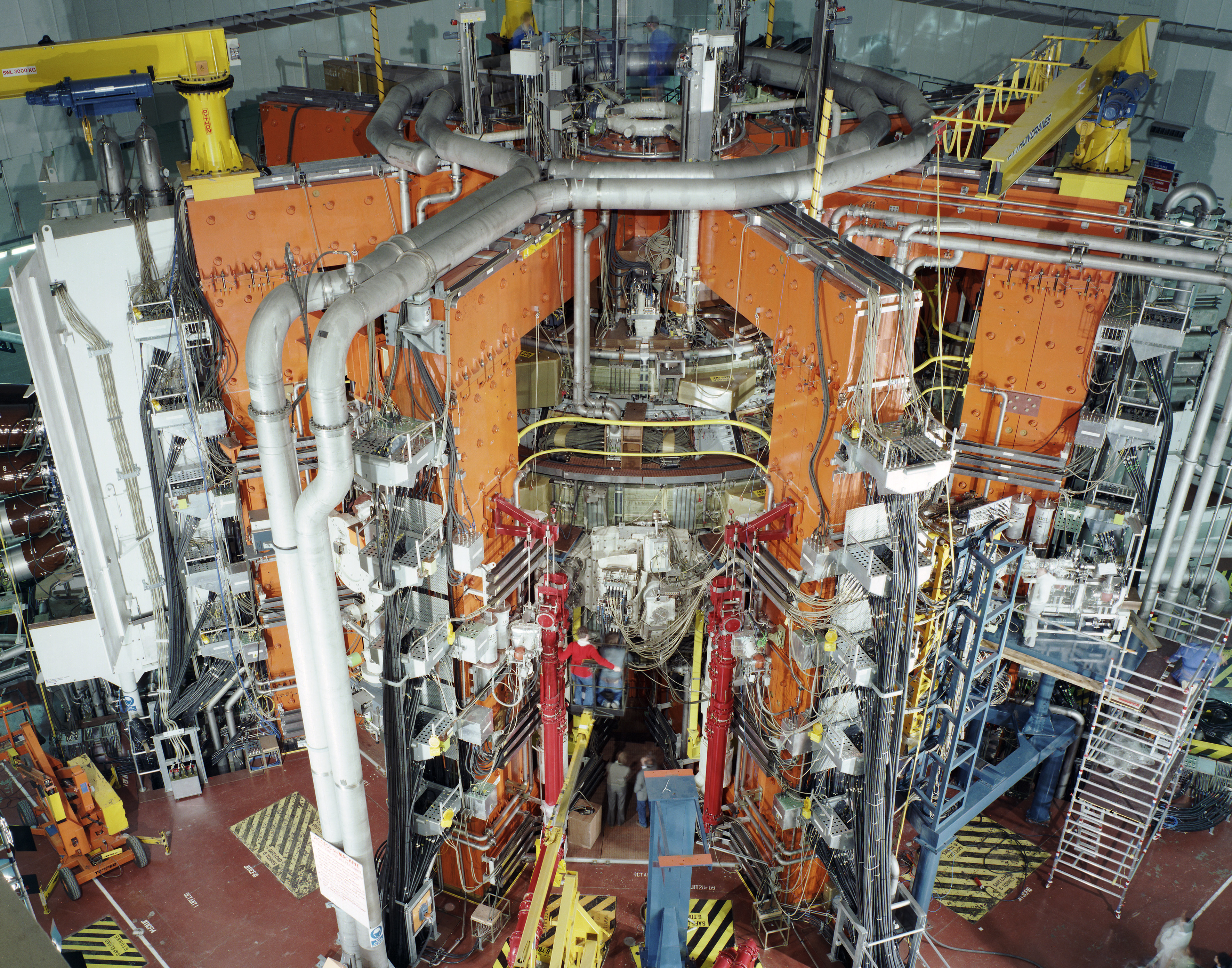
Cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: January 26, 1932, granted: February 20, 1934 A cyclotron accelerates charged particles outwards from the center of a flat cylindrical vacuum chamber along a spiral path. The particles are held to a spiral trajectory by a static magnetic field and accelerated by a rapidly varying electric field. Lawrence was awarded the 1939 Nobel Prize in Physics for this invention. The cyclotron was the first "cyclical" accelerator. The primary accelerators before the development of the cyclotron were electrostatic accelerators, such as the Cockcroft–Walton accelerator and Van de Graaff generator. In these accelerators, particles would cross an accelerating electric field only once. Thus, the energy gained by the particles was limited by the maximum elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single-photon Emission Computed Tomography
Single-photon emission computed tomography (SPECT, or less commonly, SPET) is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera (that is, scintigraphy), but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required. The technique needs delivery of a gamma-emitting radioisotope (a radionuclide) into the patient, normally through injection into the bloodstream. On occasion, the radioisotope is a simple soluble dissolved ion, such as an isotope of gallium(III). Most of the time, though, a marker radioisotope is attached to a specific ligand to create a radioligand, whose properties bind it to certain types of tissues. This marriage allows the combination of ligand and radiopharmaceutical to be carried and bound to a place of interest in the body, where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |